Show how you might use an enamine reaction to prepare each of the followingcompounds: (b) (a) CH2CH2CO2CH3
Question:
Show how you might use an enamine reaction to prepare each of the followingcompounds:
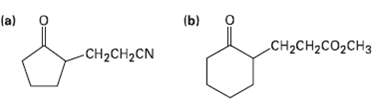
Transcribed Image Text:
(b) (a) CH2CH2CO2CH3 CH2CH2CN
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 45% (11 reviews)
Analyze the product for the Michael acceptor and the ...View the full answer

Answered By

Robert Kiptanui
I am persiuing my B tech from Maulana Azad National Institute of Technology. I am teaching student from past 3 years . I am a part of an NGO named "AAROHA" this is a firm which
give education to financially unstable students.my hobbies are problem solving and article writing. Problem-solving is an essential skill that is valuable in many areas of life, whether it be in academics, business, or personal endeavors. It involves using critical thinking and logical reasoning to find solutions to complex issues. Article writing, on the other hand, is a great way to communicate your ideas and thoughts to a wide audience. It allows you to share your knowledge and expertise on a particular topic and to engage with others who have similar interests.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show how you might use thioacetal formation and Raney nickel desulfurization to convert: (a) cyclohexanone to cyclohexane and (b) benzaldehyde to toluene.
-
Show how you might use a nucleophilic substitution reaction of 1-bromopropane to synthesize each of the following compounds. (You may use any other compounds that are necessary.) (a) (b)...
-
Using a Stork enamine synthesis, show how you might accomplish each of the following transformations. (a) (b) (c) H
-
Strickland Co. currently charges manufacturing over-head costs to products using machine hours. However, company management believes that the use of ABC would provide more realistic cost estimates...
-
Is it possible to organize too much to meet the needs of the environment? This would be a case of strategic misfit. How would you know if a misfit has occurred? Think of an example of misfit caused...
-
Since the early 1990s, woodstove sales have declined from 1,200,000 units per year to approximately 100,000 units per year. The decline has occurred because of (1) stringent new federal EPA...
-
What methods can be used to help ensure that management is selecting the right person for the position? LO.1
-
Mike Polanski is 30 years of age and his salary next year will be $40,000. Mike forecasts that his salary will increase at a steady rate of 5% per annum until his retirement at age 60. a. If the...
-
What is the total stockholders' equity based on the following account balances? $1213000. $1087000. $891000. $1066000
-
1. Why do you think Rolls has continued to bear this structural currency mismatch so long? Why hasn't it done what many automobile companies have done, and move some of their manufacturing and...
-
What products would result after hydrolysis from reaction of the enamine prepared from cyclopentanone and pyrrolidine with the following , -unsaturated acceptors? (a) CH 2 =CHCO 2 Et (b) H 2 C=CHCHO...
-
What product would you expect from a Robinson annulations reaction of 2-methyl-1, 3-cyclopentanedione with3-buten-2-one? -CH + H%3DH 2-Methyl-1,3-cyclo- pentanedione 3-Buten-2-one
-
The lengths (in minutes) of a random selection of popular childrens animated films are listed below. Estimate the true mean length of all childrens animated films with 95% confidence. 93 83 76 92 77...
-
At March 31, account balances after adjustments for Vizzini Cinema are as follows: Account Balances Accounts Cash Supplies Equipment (After Adjustment) $11,000 4,000 50,000 Accumulated...
-
2. "A student holds a thin aluminum pie pan horizontally 2 m above the ground and releases it. Using a motion detector, she obtains the graph shown in Figure P3.12. Based on her measurements, (a)...
-
Mark has two sticks, 25 inches, and 20 inches. If he places them end-to-end perpendicularly, what two acute angles would be formed when he added the hypotenuse?
-
A wedding website states that the average cost of a wedding is $29,205. One concerned bride hopes that the average is less than reported. To see if her hope is correct, she surveys 36 recently...
-
2. (10 pts each) Use partial fractions decomposition and the tables to find the inverse z- transform of each of the following: a. X(z)= 6z-z z3-4z2-z+4 4z2 b. G(z)=- (z-1) (z-0.5) 3z +1 c. X(z) =...
-
Managers cannot terminate employees who are in their beginning probationary period of work in at-will-employment states. A. True B. False
-
On April 29, 2015, Auk Corporation acquires 100% of the outstanding stock of Amazon Corporation (E & P of $750,000) for $1.2 million. Amazon has assets with a fair market value of $1.4 million (basis...
-
Ethanol is a possible fuel. Use average bond energies to calculate H rxn for the combustion of ethanol. CH3CHOH(g) + 3 O(g) 2 CO(g) + 3 HO(g)
-
(a) Give two syntheses for (CH3)2CH - O - CH2CH3, and explain which synthesis is better. (b) A student wanted to synthesize methyl tert-butyl ether, CH3 - O - C (CH3)3. He attempted the synthesis by...
-
When ethyl bromide is added to potassium tert-butoxide, the product is ethyl tert-butyl ether. (a) What happens to the reaction rate if the concentration of ethyl bromide is doubled? (b) What happens...
-
When tert-butyl bromide is heated with an equal amount of ethanol in an inert solvent, one of the products is ethyl tert-butyl ether. (a) What happens to the reaction rate if the concentration of...
-
BUS 280 Week 9 Assignment This week the assignment is about financial management. You will prepare a Cash Flow Statement for Clark's Sporting Goods and then you will calculate ratios for Sam's Paint...
-
Ayayai Restaurant's gross payroll for April is $46,800. The company deducted $2,551 for CPP$739 for Eland $9,026 for income taxes from the employeeschequesEmployees are paid monthly at the end of...
-
44. Dryer Companys policy is to keep 25% of the next month's sales in ending inventory. If Dryer meets its ending inventory policy at the end of April and sales are expected to be 24,000 units in May...

Study smarter with the SolutionInn App


