Show the 2p orbital's, and indicate the orbital overlap symbolized by the resonance structures for the carbocation
Question:
Show the 2p orbital's, and indicate the orbital overlap symbolized by the resonance structures for the carbocation in Eq. 15.32 on p. 711.
Eq. 15.32
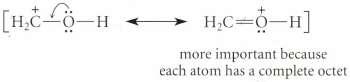
Transcribed Image Text:
more important because each atom has a complete octet
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (8 reviews)
The electrondeficient carbon has ...View the full answer

Answered By

Rohith Bellamkonda
I am studying in IIT Indore,the most prestigious institute of India.I love solving maths and enjoy coding
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
When an electron occupies a 2s orbital on an N atom it has a hyper fine interaction of 55.2 mT with the nucleus. The spectrum of N02 shows an isotropic hyper fine interaction of 5.7 mT. For what...
-
When an electron occupies a 2s orbital on an N atom it has a hyperfine interaction of 55.2 mT with the nucleus. The spectrum ofN02 shows an isotropic hyperfine interaction of 5.7 mT. For what...
-
The diatomic molecule OH exists in the gas phase. OH plays an important part in combustion reactions and is a reactive oxidizing agent in polluted air. The bond length and bond energy have been...
-
The following questions deal with the topics of internal controls and audit strategies. Required: Select and justify the audit strategy you would choose in each of the following situations. Comment...
-
The warrants of Microsystems Corporation allow the holder to buy a share of stock at $15.50 and are selling for $4.80. The stock price is currently $13.70. To what price must the stock go for the...
-
A year ago, Robin plc invested in a machine to improve the manufacturing of one of its products. It has just discovered that a new machine has come onto the market which would improve performance...
-
List the tools and techniques for performing risk control. LO.1
-
Given the contribution made on each of the three products in the following table and their position in the life cycle, identify a reasonable operations strategy foreach: Company Contribation Product...
-
Hasbro has provided the following partial listing of costs incurred during August. Assume all depreciation costs are based on straight-line depreciation. Direct Materials Indirect Materials Direct...
-
The data file agstrat.dat also contains information on other variables. For each of the following quantities, plot the data, and estimate the population mean for that variable along with its standard...
-
In each of the following sets, show by the curved-arrow or fishhook notation how each resonance structure is derived from the other one, and indicate which structure is more important and why. |.-N ]...
-
Using resonance arguments, state which ion or radical within each set is more stable. Explain. CHj HC-C CH2 or HC CH CH CH2
-
When flying at the minimum steady flight speed in level flight, if the aircraft enters a level turn without increasing its speed, the aircraft will _____ as the angle of attack is increased to...
-
1. Do you think that the NFL and franchise owners are meeting their obligations to employee health and safety? 2. Do you think that the NFL's and owners' responsibilities in terms of player safety...
-
Explain the term \'management\'. Also, explain briefly mission functions of management. ( b ) What are the different types of plant layout? Explain any two with neat sketches.
-
Suppose that you are considering an investment product that promises to pay $ 2 , 0 0 0 at the end of each year for the next five years. Assume that a discount rate of 1 2 % is applicable to similar...
-
Leadership Philosophy: Democratic and Transformational leadership In 700+ words ,explain how the leadership philosophy might impact an organization and how it would be beneficial.Identify what are...
-
performance and participation. The employee requirement that is met is status and recognition. The performance result is awakened drives. This model is dependent on leadership strive. It gives a...
-
How are KPIs and dashboards used in financial condition analysis?
-
All of the following assets can be depreciated, except: (a) A bulldozer (b) A copper mine (c) A surgical robot (d) A conveyor belt
-
We said in Section 21.6 that mechanistic studies on ester hydrolysis have been carried out using ethyl propanoate labeled with 180 in the ether- like oxygen. Assume that 180-labcled acetic acid is...
-
Treatment of a carboxylic acid with trifluoroacetic anhydride leads to an unsymmetrical anhydride that rapidly reacts with alcohol to give an ester. (a) Propose a mechanism for formation of the...
-
Treatment of an ?-amino acid with DCC yields a 2, 5-diketopiperazine. Propose a mechanism. H3N DCC H- N-H 4-N An a-amino acid A 2,5-diketopiperazine R.
-
An estimated 84 percent of enterprises now use cloud computing solutions involving multiple clouds, whereas less than 10 percent of large organizations employ just a single public cloud. Group of...
-
XYZ inc. was involved in a tax dispute with the national tax authority. The companys legal counsel estimates that there is a 75% likelihood that the company will lose the dispute and that the amount...
-
3 . Accounting.. How does depreciation impact financial statements, and what are the different methods of depreciation?

Study smarter with the SolutionInn App


