Show the structures of the likely fragments you would expect in the mass spectra of the followingmolecules:
Question:
Show the structures of the likely fragments you would expect in the mass spectra of the followingmolecules:
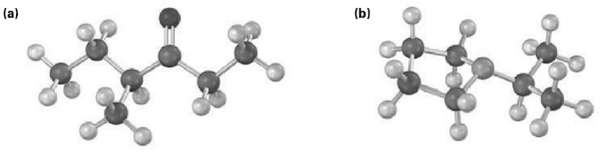
Transcribed Image Text:
(a) (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 62% (16 reviews)
a The mass spectrum of this ketone shows fragments resulting fro...View the full answer

Answered By

GERALD KAMAU
non-plagiarism work, timely work and A++ work
4.40+
6+ Reviews
11+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Show the structures of the carbocation intermediates you would expect in the following reactions: (b) CH3 (a) CH3CH2CCHCH3 HI CH H Er
-
Show the structures of the polymers that could be made from the following monomers (yellow-green =Cl): (b) (a)
-
Show the structures of all possible acid-catalyzed dehydration products of the following. If more than one alkene is possible, predict which one will be formed in the largest amount. a....
-
A 25,000 kW turbo-generator is supplied with 128,000 kg/h of steam at 2.50 MPa and 400C when developing it rated load. There are actually extracted 10,400 kg h at 0.3 MPa and 8300 kg/h at 0.06 MPa....
-
Recall from Chapter 2 Adam Smith's dictum, "The division of labor is limited by the extent of the market." How does market growth affect the viability of a focus strategy?
-
It has recently become possible to weigh DNA molecules by measuring the influence of their mass on a nano-oscillator. FIGURE P15.54 shows a thin rectangular cantilever etched out of silicon (density...
-
Suppose Consumer Reports would like to conduct a study comparing the prices of televisions made by different manufacturers. The following data show the prices of a random sample of televisions for...
-
Why are property rights so important in creating wealth?
-
What is the likely advantage of extending credit to customers? Lower accounts receivable Increased sales Reduced amount owed to creditors Fewer expenses
-
From the following information calculate the number of sales if it is desired to earn a profit of (a) $6000 or (b) $10000. Fixed cost=$12000. Selling price =$12 per unit. Variable cost=$9 per unit.
-
Where in the IR spectrum would you expect each of the following molecules toabsorb? (a) (c) (b)
-
Propose structures for compounds that fit the following mass-spectral data: (a) A hydrocarbon with M + = 132 (b) A hydrocarbon with M + = 166 (c) A hydrocarbon with M + = 84
-
The 1-year risk-free interest rate in Mexico is 10 percent. The 1-year risk-free rate in the United States is 2 percent. Assume that interest rate parity exists. The spot rate of the Mexican peso is...
-
Solve for "C" and "E": 1) E cos (15)-C=0 2) -300+ C+E sin (15) = 0
-
Let u=3, b. Compute uv, uv, 2-3 v =
-
Using Complex Numbers show that d cosz=-sinz dz
-
use for loops to solve the following problems 1. Write a complete C++ program that does the following. It asks the user to enter their age (which is assumed to be a positive integer). The program...
-
Profile Vickers hardness test Penetrating body: Square diamond pyramid :Test force F N ... 981 N (HV 5 ... HV 100) 49 :Measured value Diagonals of the square impression d Hardness value: F 0,189 F...
-
A researcher who has collected primary data from a sample of eastern European economic refugees in London wishes to confirm the validity of her conclusions concerning the pay discrimination these...
-
True & False The basis of an asset must be reduced by the depreciation allowable, 2. Adjusted gross income (AGI) is the basis for a number of phase-outs of deductions. 3. A change to adjusted gross...
-
A solution is prepared by dissolving 11.60 g of a mixture of sodium carbonate and sodium bicarbonate in 1.00 L of water. A 300.0 cm 3 sample of the solution is treated with excess HNO 3 and boiled to...
-
Predict the major product(s) of the following reactions: (a) phenylacetylene + 2 HBr (b) hex-1-yne + 2 HBr (c) cyclooctyne + 2 HCl (d) hex-2-yne + 2 HCl + 2 HBr
-
The 2 radical is more stable than 1. The anti-Markovnikov orientation occurs because the bromine radical attacks first to make the most stable radical, in contrast to electrophilic addition where the...
-
Show how hex-1-yne might be converted to (a) 1,2-dichlorohex-1-ene (b) 1-bromohex-1-ene (c) 2-bromohex-1-ene (d) 1,1,2,2-tetrabromohexane (e) 2-bromohexane (f) 2,2-dibromohexane
-
You have $55,000. You put 15% of your money in a stock with an expected return of 10%, $38,000 in a stock with an expected return of 18%, and the rest in a stock with an expected return of 22%. What...
-
Portfolio return and beta Personal Finance Problem Jamie Peters invested $ 1 1 3 , 0 0 0 to set up the following portfolio one year ago: a . Calculate the portfolio beta on the basis of the original...
-
. Emerson Cammack wishes to purchase an annuity contract that will pay him $7,000 a year for the rest of his life. The Philo Life Insurance Company figures that his life expectancy is 20 years, based...

Study smarter with the SolutionInn App


