Show the structures of the products you would obtain by hydroboration/oxidation of the following alkenes: H (a)
Question:
Show the structures of the products you would obtain by hydroboration/oxidation of the following alkenes:
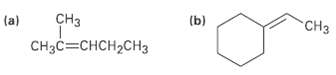
Transcribed Image Text:
сHз (a) "CHз (a) CH3C=CHCH2CH3 (b)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (20 reviews)
Strategy Hydroborationoxidation occurs with nonMarkovn...View the full answer

Answered By

Branice Buyengo Ajevi
I have been teaching for the last 5 years which has strengthened my interaction with students of different level.
4.30+
1+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Give the structures of the products you would expect when each alcohol reacts with (1) HCI, ZnCI2; HBr; (3) PBr3: (4) P/I2; (5) SOCI2- (a) butan-1-ol (b) 2-methylbutan-2-ol (c) 2,2-dimethylbutan-1-ol...
-
Show the structures of alkenes that give the following products on oxidative cleavage with KMnO4 in acidic solution: (b) (CH])2 + |(a) CHCH2CO2 co2 CH3CH2CH2CO2H (d) (c) (CH3)2C=0 CCH-CCH2CH2CHCH2CO2H
-
Show the stereochemistry of the epoxide you would obtain by formation of a bromohydrin from trans-2-butene, followed by treatment with base.
-
(a) A circular diaphragm 60 cm in diameter oscillates at a frequency of 25 kHz as an underwater source of sound used for submarine detection. Far from the source, the sound intensity is distributed...
-
A random sample of California residents who had recently visited a car dealership were asked which type of vehicle they were most likely to purchase, with the results shown. Research question: At =...
-
AI Quick, president of a Toronto Stock Exchange-listed firm , is very short-term oriented and interested in the immediate consequences of his decisions. Assume Mr. Quick is considering a project that...
-
36. On May 1, year 1, Anna received 5,000 shares of restricted stock from her employer, Jarbal Corporation. On that date, the stock price was $5 per share. On receiving the restricted stock, Anna...
-
What examples of primary research that Embraces founders completed appear in the case?
-
Question text If the required return is less than the coupon rate, a bond will sell at
-
A startup Internet company has generated the following cash balance for the first six years of its IS projects: -$250,000, -$180,000, $225,000, $340,000, $410,000, and $425,000. Using the NPV...
-
What alkenes might the following alcohols have been prepared from? (b) (a) CH3CCH2CH2CH2CH3 C
-
What alkenes might be used to prepare the following alcohols by hydroboration/oxidation? (a) CH3 (b) CHH (c) .CH- CHCHCH2CH2OH
-
Data was stored in the Digital PDP-9 computer using six-digit octal notation. Negative numbers were stored in 8s complement form. a. How many bits does six-digit octal represent? Show that 8s...
-
Claxton, Inc. paid a dividend of $ 0 . 9 5 per common share every December from 2 0 0 9 through 2 0 2 3 . The dividend is expected to continue at that level in 2 0 2 4 and 2 0 2 5 . In 2 0 2 6 and...
-
1. Who are two (2) specific examples of effective leaders (who you know personally) who have impacted your life? 2. What made them "effective" leaders? What many specific traits did these leaders...
-
pr Hwk12 Consider the differebtiable function F(x) whose instantaneous rates are given by the table of values below: I 3.00 3.50 4.00 4.50 5.00 5.50 6.00 6.50 7.00 F'(x) 21.00 27.50 35.00 43.50 53.00...
-
A stone was dropped off a cliff and hit the ground with a speed of 152 ft/s. What is the height of the cliff? (Use 32 ft/s for the acceleration due to gravity.) Step 1 We know that s(t) = 1 at + vot...
-
The 150 m long beam is submitted to a distributed load w(x) = (0.05 x 2) + 10 N/m. 50 50 w(x) 100 150 What is the moment about the point O in kN.m created by the distributed load? O-25.7 kN.m O-249...
-
How might the cost of responsibility accounting reports affect the extent to whicha formal responsibility accounting system might be instituted? LO.1
-
If the cylinder described in Problem 21.3 were initially heated to 500F, how long would it take for the center of the cylinder to cool to 240F if it were constructed of a. Copper? b. Brass? c. Nickel?
-
How is the polarity of a liquid generally related to its miscibility with water?
-
Give the structure of every stereoisomer of I,2,3- trimethylcyclohexane. Label the enantiomeric pairs and show the plane of symmetry in each a chiral stereoisomer.
-
For each of the following compounds, draw the two chair conformations that are in equilibrium. Cis- I,3-dimethylcyclohexane
-
It has been argued that the energy difference between crs- and trans-1,3-di-tert-butylcyclohexane is a good approximation for the energy difference between the chair an4 twist-boat forms of...
-
.Is bankruptcy on the part of the borrower a common risk that frequently interferes with a lenders efforts to work out a defaulted loan through either nonforeclosure means or foreclosure? Discuss.
-
For each of the following, compute the future value: Present Value Years Interest Rate $ 1 , 2 5 0 1 9 1 2 % $ 9 8 , 7 2 7 1 5 1 3 % $ 6 2 5 6 1 2 % $ 1 1 7 , 6 2 2 7 1 6 % 2 . For each of the...
-
Only need help on 4B and 5. Exercise 9-21 Breakeven Planning; Profit Planning (LO 9-2, 9-3] Connelly Inc., a manufacturer of quality electric ice cream makers, has experienced a steady growth in...

Study smarter with the SolutionInn App


