Show the terminal alkyne and alkyl halide from which the following products can he obtained. If two
Question:
Show the terminal alkyne and alkyl halide from which the following products can he obtained. If two routes look feasible, list both.
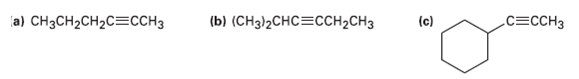
Transcribed Image Text:
a) CHзCH2CH2CEССHЗ (b) (CHз/2СHCССH-CH3 (c) СЕССНЗ
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 61% (18 reviews)
Strategy Remember that the alkyne must be a terminal alkyne and the halide must be pri...View the full answer

Answered By

Carly Cimino
As a tutor, my focus is to help communicate and break down difficult concepts in a way that allows students greater accessibility and comprehension to their course material. I love helping others develop a sense of personal confidence and curiosity, and I'm looking forward to the chance to interact and work with you professionally and better your academic grades.
4.30+
12+ Reviews
21+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
The halogen atom of an alkyl halide can be replaced by the hydrogen atom bonded to tin in tributyltin hydride (Bu3SnH). The process, called dehalogenation, is a radical reaction, and it can be...
-
The following compound cannot be prepared from an alkyl halide or a carboxylic acid using the methods described in this section. Explain why each synthesis cannot be performed. `NH2
-
The following alkyl halide can be prepared by addition of HBr to two different alkenes. Draw the structures of both (reddish brown = Br).
-
Comparative financial statements of the Boeckman Company for 2009 and 2010 are as follows: Comparative Balance Sheets Comparative Income Statements Additional information: The Boeckman Company is...
-
A given stock is currently priced at $100. Historically, its annual return has been 12 percent with a standard deviation of 15 percent. Build a spreadsheet simulation model for the stock price, using...
-
Show that the units A m 2 and J/T for the Bohr magneton are equivalent.
-
Review the information for using a power/interest grid. Find at least two articles that describe this grid or a similar one to help categorize stakeholders and how to manage them. Summarize your...
-
Williams Glassware has estimated, at various debt ratios, the expected earnings per share and the standard deviation of the earnings per share as shown in the following table. a. Estimate the optimal...
-
21 percent. What is the company's aftertax cost of debt? Multiple Choice 3.47% 4.46% 3.71% 5.87% 5.11%
-
The following accounts are taken from Foresters, Inc., a company that specializes in recovering from cognitive problems, as of December 31. Foresters, INC. Unadjusted Trial Balance At December 31...
-
The pK a of acetone, CH 3 COCH 3 , is 19.3. Which of the following bases is strong enough to de-protonate acetone? (a) KOH (p K a of H 2 O = 15.7) (b) Na + C CH (p K a of C 2 H 2 = 25) (c) NaHCO 3...
-
How would you prepare ds-2-butene starting from propyne, an alkyl halide, and any other reagents needed? This problem cant be worked in a single step. Youll have to carry out more than one reaction.
-
What is meant by cost accounting records, and what is their importance in the conduct of an audit?
-
Turn this information into an excel sheets with the excel formulas being shown P10.1 (LO 1) (Depreciation for Partial Period-SL, SYD, and DDB) Alladin Company purchased Machine #201 on May 1, 2025....
-
You are the Financial Analyst at Wellington Laboratories Ltd., a New Orleans, USA based bulk drugs manufacturer, which is evaluating the following project for manufacturing a new compound. Year Cash...
-
A variable mesh screen produces a linear and axisymmetric velocity profile as indicated below in the air flow through a 2-ft diameter circular cross section duct. The static pressures upstream and...
-
A vertical round steel rod 2 m long is securely held at its upper end. A weight can slide freely on the rod and its fall is arrested by a stop provided at the lower end of the rod. When the weight...
-
8) Determine the magnitudes of the forces F and P so that the single equivalent couple (i.e. the resultant of the three couples) acting on the triangular block is zero. Z -F F 3 m 10 N, 30 6 m 10 N 3...
-
Research careers in the gaming entertainment industry. Are there more opportunities than you realized? What careers in the industry interest you the most?
-
A. Select a recent issue (paper or online) of Report on Business Magazine, Canadian Business Magazine (online only), Bloomberg Businessweek, Fast Company, The Economist, or another business magazine....
-
Complete and balance each acidbase equation. a. HSO4(aq) + Ca(OH)2(aq) b. HClO4(aq) + KOH(aq) c. HSO4(aq) + NaOH(aq)
-
Carry out an orbital symmetry analysis to show that suprafacial [1,5] carbon migrations should occur with retention of configuration in the migrating group.
-
What product(s) are expected from a similar reaction of 2,3-dimethyl-l,3-cyclopentadiene?
-
What starting material would give the following compound in an aliphatic Claisen rearrangement? CH,-CH-C
-
ABC Company engaged in the following transaction in October 2 0 1 7 Oct 7 Sold Merchandise on credit to L Barrett $ 6 0 0 0 8 Purchased merchandise on credit from Bennett Company $ 1 2 , 0 0 0 . 9...
-
Lime Corporation, with E & P of $500,000, distributes land (worth $300,000, adjusted basis of $350,000) to Harry, its sole shareholder. The land is subject to a liability of $120,000, which Harry...
-
A comic store began operations in 2018 and, although it is incorporated as a limited liability company, it decided to be taxed as a corporation. In its first year, the comic store broke even. In...

Study smarter with the SolutionInn App


