Complete and balance each acidbase equation. a. HSO4(aq) + Ca(OH)2(aq) b. HClO4(aq) + KOH(aq) c. HSO4(aq) +
Question:
Complete and balance each acid–base equation.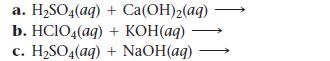
Transcribed Image Text:
a. H₂SO4(aq) + Ca(OH)2(aq) b. HClO4(aq) + KOH(aq) c. H₂SO4(aq) + NaOH(aq)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (3 reviews)
a HSO4aq CaOHaq b ...View the full answer

Answered By

Muhammad Mahtab
everyone looks that their work be perfect. I have more than a five year experience as a lecture in reputable institution, national and international. I provide perfect solution in marketing, case study, finance problems, blog writing, article writing, business plans, strategic management, human resource, operation management, power point presentation and lot of clients need. Here is right mentor who help clients in their multi-disciplinary needs.
5.00+
3+ Reviews
14+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Procedures Experiment 1: Standardize the Iodine Solution Part 1: Prepare the Materials Take a 100 mL volumetric flask from the Containers shelf and place it on the workbench. Take ascorbic acid from...
-
Procedures Experiment 1: Standardize the Iodine Solution Part 1: Prepare the Materials Take a 100 mL volumetric flask from the Containers shelf and place it on the workbench. Take ascorbic acid from...
-
First, complete and balance each of the equations below. Then, choosing among ethanol, hexane, and liquid ammonia, state which (there may be more than one) might be suitable solvents for each of...
-
Accurate Job Costing must be done on three levels. Which of the following is not one of these levels? Tracking and controlling costs during jobs Tracking gross profit each month Filing records on...
-
Why are the COSO and COBIT frameworks so important?
-
Consider the TV. What makes it so easy to use? (Almost everybody seems able to watch it.) If you were to redesign anything, what would it be, and how would you redesign it?
-
Based on your experience, what are the most significant mistakes that group trainers make as they present their training programs? (I want to assure that these topics are discussed in a...
-
Racine Tire Co. manufactures tires for all-terrain vehicles. The tires sell for $60 and variable cost per tire is $30; monthly fixed cost is $450,000. a. What is the break-even point in units and...
-
Q1 Which of the following investments is considered to have the least liquidity? Three Year Certificate of Deposit (CD). 90-Day U.S. Treasury Bill. Two Year Certificate of Deposit (CD). One Year...
-
Required Tasks: 1. Assuming that training can affect the average time but not the standard deviation, the managers are interested in knowing to what level the mean call time needs to be reduced in...
-
Write balanced molecular and net ionic equations for the reaction between nitric acid and calcium hydroxide.
-
Write balanced molecular and net ionic equations for the reaction between hydrobromic acid and potassium hydroxide.
-
You just received a $5,000 bonus. a. Calculate the future value of $5,000, given that it will be held in the bank for five years and earn an annual interest rate of 6 percent. b. Recalculate part (a)...
-
In 2022, Andrew, who is single, has a comfortable salary from his job as well as income from his investment portfolio. However, he is habitually late in filing his federal income tax return. He did...
-
1. What is the cost of direct materials used? 2. What is the cost of indirect materials used? 3. What is the cost of direct labour? 4. What is the cost of indirect labour? 5. What is the cost of...
-
Finding Critical Values. In Exercises 5-8, find the critical value za/2 that corresponds to the given confidence level. 5. 90% 6. 99%
-
You are an attorney at the law firm that represents Danfield's Auto Express. Your supervisor, Attorney Donna Defense, wants you to draft an internal memorandum of law to her assessing whether or not...
-
I desperately need help in this assignment, please help me!! Case Study Assignment You have recently been recruited by Velvet Chocolates Lid, a chocolate manufacturer, as an assistant management...
-
Consider an S-590 alloy component (Figure 8.32) that is subjected to a stress of 200 MPa (29,000 psi). At what temperature will the rupture lifetime be 500 h?
-
Reduction in sales All of the above 29. Belt of an electric motor is broken, it needs a. Corrective maintenance b. Scheduled maintenance c. Preventive maintenance d. Timely maintenance. 30. The...
-
Calculate the weight of a body in lb if it has a kinetic energy of 38.6 ft-lb when moving at 19.5 mi/h. The formula for kinetic energy is KE = mv 2 , where m = mass and v = velocity.
-
Calculate the velocity in ft/s of a 30-lb object if it has a kinetic energy of 10 ft-lb. The formula for kinetic energy is KE = mv 2 , where m = mass and v = velocity.
-
Calculate the velocity in ft/s of a 6-oz body if it has a kinetic energy of 30 in-oz. The formula for kinetic energy is KE = mv 2 , where m = mass and v = velocity.
-
Penske Ltd has a standard deviation of returns of 18% and a correlation with the market portfolio of 0.8. The market portfolios expected return is 14%, its standard deviation of returns is 12%, and...
-
In an insurance policy identification of the named insured is important in terms of determining who is the insured. In the body of the policy the names insured oftentimes is referred to as "you"....
-
During the year Salaries payable decreased by $6,000. If Salary expense amounted to $160,000 for the year, the cash paid to employees is: Select one: a. $160,000. b. $172,000. c. $166,000. d....

Study smarter with the SolutionInn App


