A solution of a 0.0014 g of benzophenone in 1 L of ethanol has A = 0.153
Question:
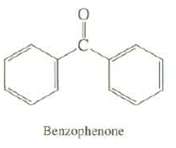
A solution of a 0.0014 g of benzophenone in 1 L of ethanol has A = 0.153 (1 cm cell) at ? max = 252 nm. Calculate the molar absorptivity of benzophenone.
Transcribed Image Text:
Benzophenone
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (10 reviews)
Acl 0153 000...View the full answer

Answered By

Vijender Singh
I have teaching experience of around 15 years, from extensive tutoring and individual mentoring to teaching school/graduates students. In my present institution, I prepare and deliver my own lectures, hold office hours and review session, designed curriculum, help to write and grade exam and am always accessible to students by email and various tutoring platform. My teaching experience includes teaching a variety of students with versatile theoretical and practical problem's solution.
I embrace every teaching opportunity that I can find, and I have worked enthusiastically and effectively with students at a variety of levels. I believe in keeping all my courses and tutoring session student-centered, and so I focus on creating a dialogue with the students and to help them discover answers for themselves. Finally, I am dedicated to enriching the lives of students outside the classroom, through student project/assignment/committee work.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
A 13.0 g wire of length L = 62.0 cm is suspended by a pair of flexible leads in a uniform magnetic field of magnitude 0.440 T (Figure). What are the (a) Magnitude and (b) Direction (left or right) of...
-
A solution of ethanol has been contaminated with benzene-a technique employed to make ethanol unfit to drink. Benzene has a molar absorptivity of 230 at 260 nm in ethanol, and ethanol shows no...
-
Part A Light has both a wave and a particle nature. Particles have a wave nature as well, and therefore All particles decay with a certain characteristic lifetime. Their position can not be specified...
-
Omer is part of the management team of a Canadian public company and is eligible for the employee stock option plan. A few years ago he received an option on 1,000 shares. The current price of the...
-
The table shows the total energy supply from crude oil products, in quadrillion BTUs, for selected years from 2010 and projected to 2040. (a) Find the cubic function that is the best model for the...
-
Ingratiation is defined as a class of strategic behaviors designed to make others believe in the attractiveness of one's personal qualities. In organizational settings, individuals use such behaviors...
-
The management of Universal Manufacturing Company (Problem 5-4) likes to have its operators working 90% of the time. What must the assembly line arrival rate be in order for the operators to be as...
-
Shares are ______ when the corporation will not issue physical stock certificates. a.Approved b.Unapproved c.Unacknowledged d.Acknowledged e.Uncertificated
-
Suppose a company wants to design a data warehouse to facilitate the analysis of moving vehicles in an online analytical processing manner. The company registers huge amounts of auto movement data in...
-
Anthracene has 1.80 x 105 M1 cm1 at max = 256nm calculate the absorbance of a 1.94 x 106M solution of anthracene in a 1 cm cell.
-
Trans-1-Phenyl-1, 3-butadiene has max = 280 ( = 27,000) calculate the concentration of a solution that has A = 0.643 at 280nm in a 1 cm cell.
-
Describe the stages of the legal writing process.
-
Contract for construction crew and equipment 8 Build parking lots Exterior lighting 11 7 20 12 Build foundation Start Interior Interior 12 9 electrical Final wiring finish Purchase 8 14 12 material...
-
Mad Hatter Enterprises purchased new equipment for $369,000, terms f.o.b. shipping point. Other costs connected with the purchase were as follows: State sales tax Freight costs Insurance while in...
-
Write down a C program that takes runs scored by a batsman and prints the status according to the following policy: Runs scored >80 50-79 30-49 10-29 <10 Grade Excellent 4 Very Good Good Average Poor
-
Consider the standard two-period maximization problem for investor j over s states of nature: Subject to S max u(c) + (s)u(c;}(s)) S=1 Cjo + q(s) C; (s) = Wjo +244) S=1 where all terms are as defined...
-
At what point should a leader cease gathering data, take the risk, and simply make the decision? Support your position.
-
Do you think the various impacts have changed in line with the change in venue from largely metropolitan to the current locations if so, how have they changed?
-
A fuel pump sends gasoline from a car's fuel tank to the engine at a rate of 5.88 10-2 kg/s. The density of the gasoline is 735 kg/m3, and the radius of the fuel line is 3.18 10-3 m. What is the...
-
In 1998, John Tauras of the University of Illinois at Chicago and Michael Grossman of the City University of New York conducted a study of teen use of cocaine. They found that compared to adults,...
-
Consider a chemical species (either a molecule or an ion) in which a carbon atom forms three single bonds to three hydrogen atoms and in which the carbon atom possesses no other valence electrons....
-
Consider a chemical species like the one in the previous problem in which a carbon atom forms three single bonds to three hydrogen atoms, but in which the carbon atom possesses an unshared electron...
-
Consider another chemical species like the ones in the previous problems in which a carbon atom forms three single bonds to three hydrogen atoms but in which the carbon atom possesses a single...
-
Eye Deal Optometry leased vision - testing equipment from Insight Machines on January 1 , 2 0 2 4 . Insight Machines manufactured the equipment at a cost of $ 2 0 0 , 0 0 0 and lists a cash selling...
-
help! ee all photos + Add to o e D C N X Edit & Create Share Table of Contents No sales to an individual customer accounted for more than 10% of revenue during any of the last three fiscal years. Net...
-
Business law A person may have the liability of a partner even though no partnership exists True False

Study smarter with the SolutionInn App


