Confirm that item (iv) in P4.2 is equal to (left(b_{1}-b_{2} ight)), where (b=h-T_{0} s), the specific steady-flow
Question:
Confirm that item (iv) in P4.2 is equal to \(\left(b_{1}-b_{2}\right)\), where \(b=h-T_{0} s\), the specific steady-flow availability function. (Note that, since \(T_{2}=T_{0}\) and \(p_{2}=p_{0}\), state 2 is the dead state, so that item (iv) is in this case equal to the steady-flow exergy of unit mass of air in state \(1 \mathrm{for}\) an environment at \(T_{0}=300 \mathrm{~K}\) and \(p_{0}=1\) bar.) \([128.2 \mathrm{~kJ} / \mathrm{kg}]\)
P4.2
Air passes slowly through a rigid control volume A, as shown in Fig. P4.2(a), in a hypothetical, fully reversible, steady-flow process between specified stable end states 1 and 2 in the presence of an environment at temperature \(T_{0}\) and pressure \(p_{0}\). States 1 and 2 , and also the values of \(T_{0}\) and \(p_{0}\), are defined below.
\[\begin{aligned}& T_{1}=550 \mathrm{~K} \text { and } p_{1}=2 \mathrm{bar} . \\& T_{2}=T_{0}=300 \mathrm{~K} \quad \text { and } p_{2}=p_{0}=1 \mathrm{bar}.\end{aligned}\]
The air may be treated as a perfect gas, with \(c_{p}=1.005 \mathrm{~kJ} / \mathrm{kg} \mathrm{K}\) and \(c_{v}=0.718 \mathrm{~kJ} / \mathrm{kg} \mathrm{K}\).
FIGURE P4.2
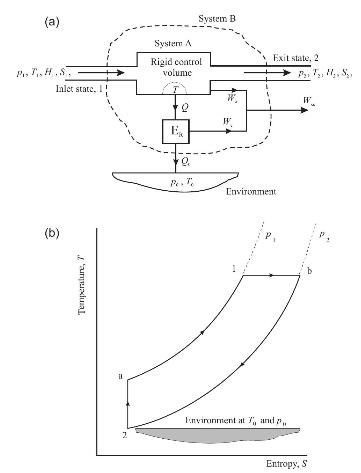
(a) When the air follows path 1-a-2, see Fig. P4.2(b), calculate the following work output quantities in each of the subprocesses 1-a and a-2.
(i) The direct shaft work, \(W_{x_{\mathrm{A}}}\), coming from the control volume as the air passes through the given subprocess.
(ii) The external work, \(W_{\mathrm{e}}\), produced by any auxiliary cyclic devices required to ensure external reversibility in that exchange with the environment during the given subprocess.
(iii) The gross work, \(W_{\mathrm{g}}\).
(iv) The total useful shaft work, \(W_{\mathrm{x}}\). [66.03; 62.2; 128.23; \(128.23(\mathrm{~kJ} / \mathrm{kg})\) ]
(b) Calculate the above work output quantities for each of the subprocesses 1-b and b-2 when the air follows the alternative path 1-b-2.
[109.41; 18.77; 128.18; \(128.18(\mathrm{~kJ} / \mathrm{kg})]\)
Step by Step Answer:

Advanced Thermodynamics For Engineers
ISBN: 9780080999838
2nd Edition
Authors: D. E. Winterbone, Ali Turan





