In the preceding problem, the composition of the feed was assumed to be constant. However, the CO/H
Question:
In the preceding problem, the composition of the feed was assumed to be constant. However, the CO/H2 ratio in the feed can be manipulated as these streams are usually supplied from different sources. Solve the preceding optimization problem by considering the CO/H2 ratio as another decision variable. Did you have to consider any additional constraints? Why? Compare the results with the results that you obtained in Problem 16.21.
Problem 16.21
In a process for producing methanol, synthesis gas composed of CO, CO1, H1, and H2O is sent through a heat exchanger, E- 2001, to a plug-flow reactor, R-2001, as shown in Figure P16.21. In addition to the desired methanol synthesis, some undesired reactions also take place: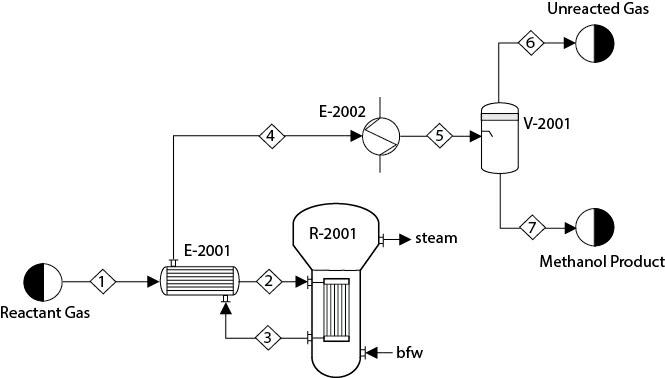
![The corresponding rate laws for these reactions are [22, 23] TCH, OH -TCO kmol kmol kg cat s CO + 2H CH3 OH](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1699/3/5/4/419654a1733733ae1699354413651.jpg)
where C is the concentration of the species i in kmol/m3.
In R-2001, steam is being generated at a temperature of 350°C. Consider an overall heat transfer coefficient of 80 W/m °C between the process stream and the boiling water. The reactor effluent is sent to E-2001 and then further cooled in E-2002 to 40°C before being sent to the flash separator, V-2001. Unconverted reactants are recovered from the top of V-2001. Assume that the feed composition (mole fraction) and rate are
The minimum temperature approach in E-2001 is 15°C. Further assume that the pressure drops in both the shell and tube sides of E-2001 are 0.1 bar. The catalyst particle density is 1800 kg/m and the bed voidage is 0.45. Assume the pressure drop in R- 2001 is 0.2 bar. Maximize the yield of methanol in this process, where the decision variables are the inlet temperature, the pressure of Stream 1, and the dimensions of R-2001 (length and diameter). What bounds on the decision variables did you consider? Why? You can solve this problem with either the SM or EO approach.
Step by Step Answer:

Analysis Synthesis And Design Of Chemical Processes
ISBN: 9780134177403
5th Edition
Authors: Richard Turton, Joseph Shaeiwitz, Debangsu Bhattacharyya, Wallace Whiting





