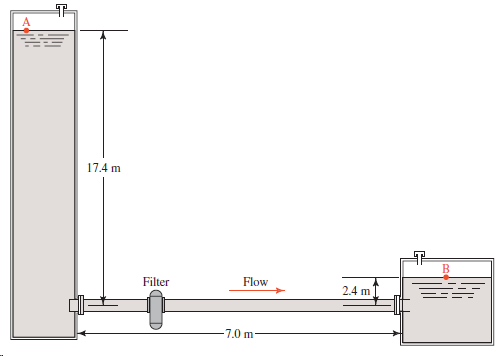
For the system described in Problem 11.47, and using the tube size found in that problem, compute
Question:
Transcribed Image Text:
17.4 m Flow Filter 2.4 m -7.0 m
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (12 reviews)
APPLIED FLUID MECHANICS Objective Volume flow rate Problem 1148 Figure 1133 Propyl alcohol at 25C Sy...View the full answer

Answered By

Joseph Njoroge
I am a professional tutor with more than six years of experience. I have helped thousands of students to achieve their academic goals. My primary objectives as a tutor is to ensure that students do not have problems while tackling their academic problems.
4.90+
10+ Reviews
27+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Suppose that for the system described in Problem 129, the spring constants are each k = 60 N/m. The system starts from rest and slowly accelerates until the masses are 0.8 m from the center of the...
-
For the pumping system described in Problem 14.23, how will the maximum elevation above the surface of the reservoir change if the water temperature is 80C(P v = 47.35 kPa)? Data From Problem 14.23 A...
-
For the steam power plant described in Problem 11.1, assume the isentropic efficiencies of the turbine and pump are 85% and 80%, respectively. Find the component specific work and heat transfers and...
-
Let (x) = x 2 + 3x + 2 and g(x) = x + 1. Find each of the following. (a) (fg)(x) (b) (fg) (-2) (c) (+)(x) (d) ()(-2)
-
Justine argues that all companies should use the revaluation model as it provides more useful information than the cost model. She is also concerned that, if companies do have a choice, they will...
-
What are the five stages of group development?
-
(a) Obtain the SE for the difference between the mean yields for the following factor levels or combinations of levels: (i) nitrogen Level 1 vs. nitrogen Level 3 (ii) variety Victory at nitrogen...
-
A 0.25-m3 insulated pistoncylinder device initially contains 0.7 kg of air at 20°C. At this state, the piston is free to move. Now air at 500 kPa and 70°C is allowed to enter the cylinder...
-
Your firm needs a computerized machine tool lathe that costs $50,000, requires $10,000 in installation, $5,000 in freight charges, and another $12,000 in maintenance for each year of its three-year...
-
Using the returns for the Bledsoe Large-cap stock fund and the Bledsoe Bond fund, graph the opportunity set of feasible portfolios. Please use excel sheet with E(r), Var(r), Cov, and Std(r). Also,...
-
For the system described in Problem 11.47, and using the tube size found in that problem, compute the expected volume flow rate through the tube if the pressure above the fluid in the large tank at A...
-
Figure 11.33 shows a part of a chemical processing system in which propyl alcohol at 25°C is taken from the bottom of a large tank and transferred by gravity to another part of the system. The...
-
The validity of evidence depends ultimately on the: a. Attestation standards and GAAS. b. Availability of subordinate evidence. c. Relevance of the evidence. d. Practitioners professional judgment....
-
Mijka Company was started on January 1, Year 1. During Year 1, the company experienced the following three accounting events: 1. earned cash revenues of $32,500 2. paid cash expenses of $14,500 3....
-
Q2. Find the equations of the tangent and normal to the curve x3 + y = 2 at (1, 1). Q3. Find if y dx y= :xsinx + (sinx)cosx [10] [10]
-
Assume you have been given $400,000 CAD with access to all listed stocks, bonds, futures, and options worldwide. You can trade in options and futures, in combination with the underlying asset....
-
The formula weight (FW) of a gas can be determined using the following form of the ideal gas law FW = g R T / PV where g is the mass in grams, R is the gas constant, T is the temperature in Kelvin, P...
-
Consider a game in which a fair die is thrown. The player pays $5 to play and wins $2 for each dot that appears on the roll. Define X = number on which the die lands, and Y = player's net profit...
-
Which of the following describes the derivative function (x) of a cubic (third degree) function (x)? (a) Quadratic (b) Linear (c) Constant (d) Cubic
-
For each of the following reactions, express the equilibrium constant: a) H20 (I) H2 (g) + 02 (g) Ke = 1.0x107 b) Fe2 (g) 2F (g) Ke= 4.9 x 10-21 c) C (s) + O2 (g) d) H2 (g) + C2H4 (g) C2H6 (g) Ke =...
-
When the following compound is treated with sodium ethoxide, two condensation products are obtained, both of which are produced via Dieckmann cyclizations. Draw both products. OEt Eto
-
For each of the following reactions, predict the major product and propose a mechanism for its formation. (a) (b) (c) :? 1) LDA 2) CH3I 1) NaH 2) -CH,Br
-
Identify the reagents you would use to achieve the following transformation:
-
The Regal Cycle Company manufactures three types of bicyclesa dirt bike, a mountain bike, and a racing bike. Data on sales and expenses for the past quarter follow: Total Dirt Bikes Mountain Bikes...
-
?? A local college is deciding whether to conduct a campus beautification initiative that would imvolve various projects, such as planting trees and remodeling bulidings, to make the campus more...
-
A company has net income of $196,000, a profit margin of 9.7 percent, and an accounts receivable balance of $135,370. Assuming 70 percent of sales are on credit, what is the companys days sales in...

Study smarter with the SolutionInn App


