Question: If gas is cooled under conditions of constant volume, it is noted that the pressure falls nearly proportionally as the temperature. If this were to
If gas is cooled under conditions of constant volume, it is noted that the pressure falls nearly proportionally as the temperature. If this were to happen until there was no pressure, the theoretical temperature for this case is referred to as absolute zero. In an elementary experiment, the following data were found for pressure and temperature under constant volume.
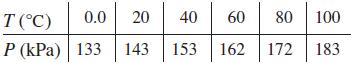
Use a calculator to find the least-squares line for P as a function of T, and from the graph determine the value of absolute zero found in this experiment.
T (C) 0.0 20 40 60 80 100 P (kPa) 133 143 153 162 172 183
Step by Step Solution
3.52 Rating (169 Votes )
There are 3 Steps involved in it
To find the leastsquares line for P as a function of T we need to calculate the slope and intercept ... View full answer

Get step-by-step solutions from verified subject matter experts


