Calculate the pH of a buffer solution that contains 0.10 M acetic acid (Table 2.6) and 0.25
Question:
Calculate the pH of a buffer solution that contains 0.10 M acetic acid (Table 2.6) and 0.25 M sodium acetate.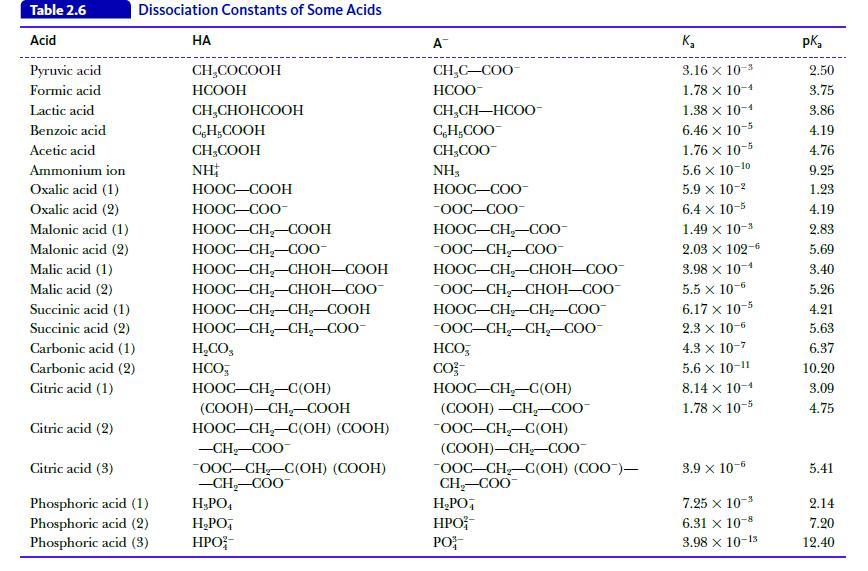
Transcribed Image Text:
Table 2.6 Acid Pyruvic acid Formic acid Lactic acid Benzoic acid Acetic acid Dissociation Constants of Some Acids Ammonium ion Oxalic acid (1) Oxalic acid (2) Malonic acid (1) Malonic acid (2) Malic acid (1) Malic acid (2) Succinic acid (1) Succinic acid (2) Carbonic acid (1) Carbonic acid (2) Citric acid (1) Citric acid (2) Citric acid (3) Phosphoric acid (1) Phosphoric acid (2) Phosphoric acid (3) HA CH₂COCOOH HCOOH CH₂CHOHCOOH CH₂COOH CH₂COOH NH HOOC–COOH HOOC—COO- HỌOC–CH,COOH HOOC–CH_–C00- HOOC–CH,CHOH—COOH HOOC–CH,–CHOH–COO- HOOC–CH–CH_–COOH HOOC–CH,CH,–COO- H,CO, HCO HOOC–CH,–C(OH) (COOH)-CH₂-COOH HOOC–CH,–C(OH) (COOH) -CH₂-COO™ OOC-CH₂-C(OH) (COOH) -CH₂-COO™ H₂PO4 H₂PO HPO A™ CH₂C-COO HCOO- CH,CH—HCOO- C, H,COO CH₂COO™ NH3 HỌỌC—COO -OOC-COO- HOOC–CH,–C0O- -OOC-CH₂-COO- HOOC–CH–CHOH–COO OOC-CH₂-CHOH-COO HOOC–CH_CH,COO -OOC-CH₂-CH₂-COO- HCO, CO²- HOOC–CH–C(OH) (COOH) -CH₂-COO™ OOC-CH₂-C(OH) (COOH)-CH₂-COO™ OOC-CH₂-C(OH) (COO)— CH₂-COO™ H₂PO4 HPO PO K₂ 3.16 x 10-3 1.78 x 10-4 1.38 x 10-4 6.46 x 10-5 1.76 x 10-5 5.6 x 10-10 5.9 x 10-² 6.4 x 10-5 1.49 × 10-³ 2.03 × 102-6 3.98 x 10-4 5.5 x 10-6 6.17 x 10-5 2.3 x 10-6 4.3 x 10-7 5.6 x 10-11 8.14 x 10-1 1.78 x 10-5 3.9 x 10-6 7.25 x 10-3 6.31 x 10-8 3.98 x 10-15 pka 2.50 3.75 3.86 4.19 4.76 9.25 1.23 4.19 2.83 5.69 3.40 5.26 4.21 5.63 6.37 10.20 3.09 4.75 5.41 2.14 7.20 12.40
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (4 reviews)
Use the HendersonHassel Bal...View the full answer

Answered By

S Mwaura
A quality-driven writer with special technical skills and vast experience in various disciplines. A plagiarism-free paper and impeccable quality content are what I deliver. Timely delivery and originality are guaranteed. Kindly allow me to do any work for you and I guarantee you an A-worthy paper.
4.80+
27+ Reviews
73+ Question Solved
Related Book For 

Biochemistry
ISBN: 9781305961135
9th Edition
Authors: Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Question Posted:
Students also viewed these Sciences questions
-
First, write the code to create a single DataFrame object in a function called load_ticket_data(). This function should return the full dataframe and take no parameters (you can assume the ticket...
-
QUESTION 4 (25 MARKS: 45 MINUTES) < Daniaa Beauty Sdn. Bhd. is a business that sells skincare to customers. The business has extended its market segment to northern part of Malaysia. In addition to...
-
Calculate the pH of a buffer solution that is 0.050 M in benzoic acid (HC 7 H 5 O 2 ) and 0.150 M in sodium benzoate (NaC 7 H 5 O 2 ). For benzoic acid, Ka = 6.5 * 10 -5 .
-
Problem Set 3 b. zero. c. negative. d. smaller than the variance. 22. Growth factors for the population of Atlanta in the past five years have been 1, 2, 3, 4, and 5. The geometric mean is a. 15. b....
-
You are the manager of a monopoly. A typical consumers inverse demand function for your firms product is P = 100 20Q, and your cost function is C(Q) = 20Q. a. Determine the optimal two-part pricing...
-
What is the chief disadvantage of ADO.NET DataSets? When is this likely to be a problem?
-
14. Assume that supplies on hand at the beginning of the year amount to $60,000 and that supply purchases during the year are $400,000. Supplies on hand at year-end are $40,000, and the consumption...
-
(a) Prepare any necessary transaction entries for 2019 and adjusting entries at December 31, 2019, using the financial statement effects template.(b) Prepare any necessary transaction entries for...
-
Barbarino Corporation purchased land and a building for $ 1 comma 7 0 0 comma 0 0 0 $ 1 , 7 0 0 , 0 0 0 . An appraisal indicates that the land's market value is $ 7 0 0 comma 0 0 0 $ 7 0 0 , 0 0 0...
-
Calculate the pH of a buffer solution prepared by mixing 75 mL of 1.0 M lactic acid (see Table 2.6) and 25 mL of 1.0 M sodium lactate. Table 2.6 Acid Pyruvic acid Formic acid Lactic acid Benzoic acid...
-
List the criteria used to select a buffer for a biochemical reaction.
-
You are the chief financial accountant of Soda plc, a manufacturer and wholesaler of soft drinks. Soda plc is in direct competition with Fizz plc and Pop Ltd. The finance director has informed you...
-
Complete the "Leadership Vision Questionnaire" in Chapter 7 (p176). Reflect on your results and complete the following prompts: Share the results from your questionnaire. Be sure to include the final...
-
1. Prepare el Presupuesto Operacional hasta completar el COGS (70 puntos) La empresa ACCO 295 tiene una venta proyectada de $450,000 Cada unidad se vende $450 Su inventario inicial 300 (costo $125)...
-
Continuing Case 65. Retirement Income Forecast Jamie Lee and Ross, now 57 and still very active, have plenty of time on their hands now that the triplets are away at college. They both realized that...
-
The partnership of Frick, Wilson, and Clarke has elected to cease all operations and liquidate its business property. A balance sheet drawn up at this time shows the following account balances: Cash...
-
Harry and Sally went to a large hardware store and told the salesperson they wanted the cheapest rotating clothesline in stock, provided it would bear a heavy load of washing. The salesperson assured...
-
The weight of a boat is listed on specification sheets as its "displacement"? Explain.
-
If there is an unrealized holding gain on available-for-sale investments, it is reported as?
-
Why is it somewhat misleading to study biochemical pathways separately?
-
What is the Warburg effect? Why would cancer cells favor such inefficient metabolism?
-
To what extent can metabolic pathways be considered reversible? Why?
-
As a long-term investment at the beginning of the 2018 fiscal year, Florists International purchased 25% of Nursery Supplies Inc.'s 18 million shares for $66 million. The fair value and book value of...
-
Javier is currently paying $1,200 in interest on his credit cards annually. If, instead of paying interest, he saved this amount every year, how much would he accumulate in a tax-deferred account...
-
Your company is considering the purchase of a fleet of cars for $195,000. It can borrow at 6%. The cars will be used for four years. At the end of four years they will be worthless. You call a...

Study smarter with the SolutionInn App


