Calculate the pH of a buffer solution prepared by mixing 75 mL of 1.0 M lactic acid
Question:
Calculate the pH of a buffer solution prepared by mixing 75 mL of 1.0 M lactic acid (see Table 2.6) and 25 mL of 1.0 M sodium lactate.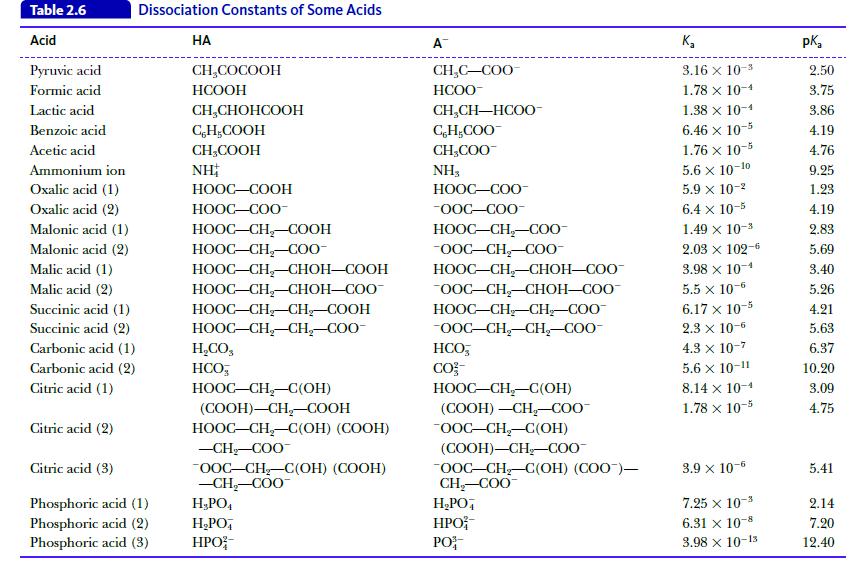
Transcribed Image Text:
Table 2.6 Acid Pyruvic acid Formic acid Lactic acid Benzoic acid Acetic acid Ammonium ion Oxalic acid (1) Oxalic acid (2) Malonic acid (1) Malonic acid (2) Malic acid (1) Malic acid (2) Succinic acid (1) Succinic acid (2) Carbonic acid (1) Carbonic acid (2) Citric acid (1) Citric acid (2) Dissociation Constants of Some Acids Citric acid (3) Phosphoric acid (1) Phosphoric acid (2) Phosphoric acid (3) HA CH₂COCOOH HCOOH CH₂CHOHCOOH CH₂COOH CH₂COOH NH HỌOC–COOH HỌOC—COO- HỌOC–CH,–COOH HOOC–CH,C00- HOOC–CH,–CHOH—COOH HOOC–CH,–CHOH–COO- HOOC–CH_CH, COOH HOOC–CH,CH,–COO- H,CO, HCO HOOC–CH,–C(OH) (COOH)-CH₂-COOH HOOC–CH,–C(OH) (COOH) -CH₂-COO OOC-CH₂-C(OH) (COOH) -CH₂-COO H₂PO4 H₂PO4 HPO A CH₂C-COO HCOO- CH,CH—HCOO- C,H,COO CH₂COO™ NH3 HỌỌC—COO- -OOC-COO- HOOC–CH,–C00- -OOC-CH₂-COO- HOOC–CH–CHOH–COO- OOC-CH₂-CHOH-COO™ HOOC–CH_CH_COO -OOC-CH₂-CH₂-COO- HCO, CO²- HOOC–CH–C(OH) (COOH) -CH₂-COO™ -OOC-CH₂-C(OH) (COOH)-CH₂-COO™ OOC-CH₂-C(OH) (COO)— CH₂-COO™ H₂PO4 HPO PO K₂ 3.16 x 10-3 1.78 x 10-1 1.38 x 10-1 6.46 x 10-5 1.76 x 10-5 5.6 x 10-10 5.9 x 10-² 6.4 x 10-5 1.49 × 10-3 2.03 × 102-6 3.98 x 10-4 5.5 x 10-6 6.17 x 10-5 2.3 x 10-6 4.3 x 10-7 5.6 X 10-11 8.14 x 10-1 1.78 x 10-5 3.9 x 10-6 7.25 x 10- 6.31 x 10-8 3.98 x 10-13 pka 2.50 3.75 3.86 4.19 4.76 9.25 1.23 4.19 2.83 5.69 3.40 5.26 4.21 5.63 6.37 10.20 3.09 4.75 5.41 2.14 7.20 12.40
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (3 reviews)
After mixing the buffer solution 100 mL contains 075 M ...View the full answer

Answered By

Chiranjib Thakur
I have no tutoring experience yet, but I can share my skills and knowledge gained from my education and work experiences. I have been a CPA since 2012 with 6 years of work experience in internal auditing and 4 years of work experience in accounting at the supervisory level.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Biochemistry
ISBN: 9781305961135
9th Edition
Authors: Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Question Posted:
Students also viewed these Sciences questions
-
Calculate the pH of a buffer solution prepared by mixing 25 mL of 1.0 M lactic acid and 75 mL of 1.0 M sodium lactate.
-
(a) Calculate the pH of a buffer that is 0.12 M in lactic acid and 0.11 M in sodium lactate. (b) Calculate the pH of a buffer formed by mixing 85 mL of 0.13 M lactic acid with 95 mL of 0.15 M sodium...
-
The Kb for NH3 is 1.8 10-5 at 25C. Calculate the pH of a buffer solution made by mixing 65.1 mL of 0.142 M NH3 with 39.2 mL of 0.172 M NH4Cl at 25C. Assume that the volumes of the solutions are...
-
Problem A (20 points): Loco Farms Company sold 36,000 units of its only product and incurred a $18,672 loss (ignoring taxes) for the current year as shown here. During a planning session for year...
-
A monopoly is considering selling several units of a homogeneous product as a single package. A typical consumers demand for the product is Qd = 50 .25P, and the marginal cost of production is $120....
-
How is referential integrity defined in ADO.NET? What referential integrity actions are possible?
-
13. Define construction contract. Explain how a construction in progress is recorded in a governmental fund.
-
Assume that Whee, Cheatham, and Howe is an auditing firm that has found that its summer interns are subject to a 90 percent learning curve for one of its important tasks, proofreading financial...
-
Hearne Company has a number of potential capital investments. Because these projects vary in nature, initial investment, and time horizon, management is finding it difficult to compare them. Assume...
-
What is the relationship between pK a and the useful range of a buffer?
-
Calculate the pH of a buffer solution that contains 0.10 M acetic acid (Table 2.6) and 0.25 M sodium acetate. Table 2.6 Acid Pyruvic acid Formic acid Lactic acid Benzoic acid Acetic acid Dissociation...
-
For each polynomial function, (a) list all possible rational zeros, (b) find all rational zeros, and (c) factor (x) into linear factors. Zeros of -2, 1, and 0; (-1) = -1
-
Prevosti Farms and Sugarhouse pays its employees according to their job classification. The following employees make up Sugarhouse's staff: Payroll Payroll Register Register Thomas Avery Towle...
-
Name: Course: Worksheet Lab Experience 5 Logic Circuits (A) Exercise 5.1 Truth table for the example circuit A B Output Value 0 0 1 1 0 1 1 Exercise 5.2 A slight change in the example circuit...
-
Stanley Medical Hospital is a non-profit and a non-chartered hospital planning to acquire several hospitals in the area. The hospital is researching financial options since they want to expand into...
-
Tony and Suzie see the need for a rugged all-terrain vehicle to transport participants and supplies. They decide to purchase a used Suburban on July 1, 2022, for $12,000. They expect to use the...
-
Pacifico Company, a US-based importer of beer and wine, purchased 1,800 cases of Oktoberfest-style beer from a German supplier for 522,000 euros. Relevant U.S. dollar exchange rates for the euro are...
-
In tall buildings, the water supply system uses multiple pumping stations on different floors. At each station, water pumped up from below collects in a storage tank held at atmospheric pressure...
-
Let X be a random variable taking on values a1, a2, . . . , pr with probabilities p1, p2, . . . , pr and with E(X) = μ. Define the spread of X as follows: This, like the standard deviation, is a...
-
Why would a runner who has a 5-km race to run at 9 am be concerned about insulin?
-
Why do some people call GLUT4 the training glucose transporter?
-
How are insulin, GLUT4, obesity, and type II diabetes related?
-
A stock is expected to pay a dividend of $1.50 at the end of the year (i.e., D 1 = $1.50), and it should continue to grow at a constant rate of 10% a year. If its required return is 14%, what is the...
-
The Hobby Shop has a checking account with a ledger balance of $1,700. The firm has $2,400 in uncollected deposits and $4,200 in outstanding checks. What is the amount of the disbursement float on...
-
An investment will pay you $34,000 in 11 years. If the appropriate discount rate is 6.1 percent compounded daily, what is the present value? (Use 365 days a year. Do not round intermediate...

Study smarter with the SolutionInn App


