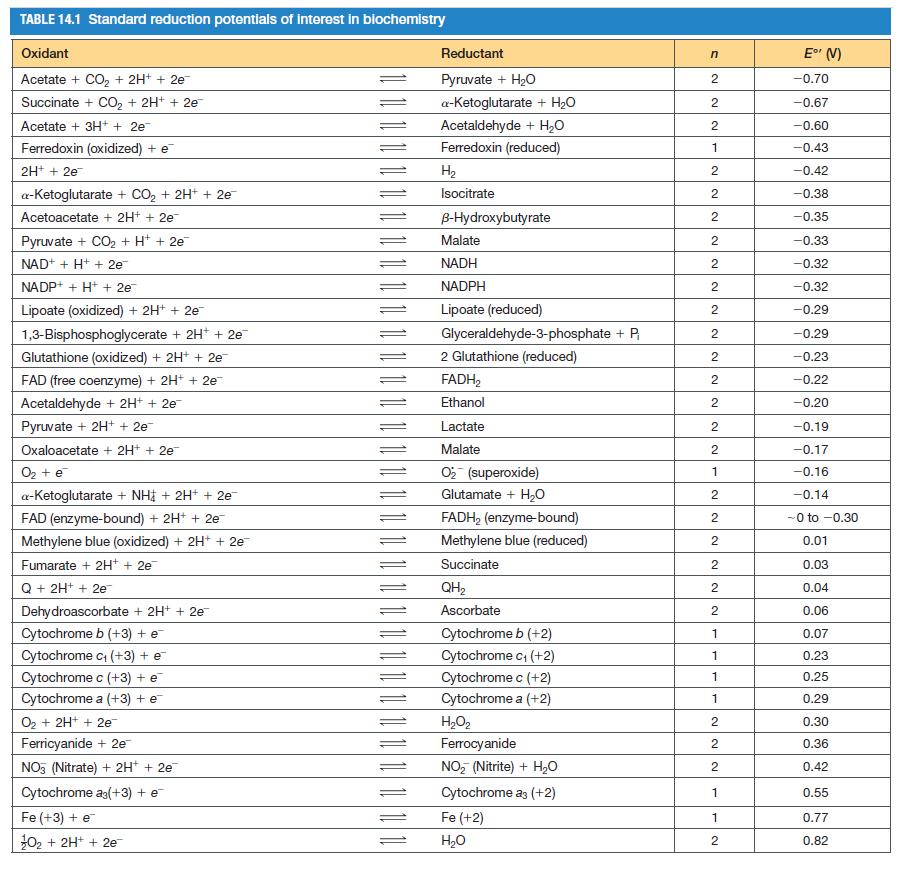
From E values in Table 14.1, calculate the equilibrium constant for the glutathione peroxidase reaction at 37
Question:
From E°′ values in Table 14.1, calculate the equilibrium constant for the glutathione peroxidase reaction at 37 °C.
Table 14.1
Transcribed Image Text:
TABLE 14.1 Standard reduction potentials of interest in biochemistry Oxidant Acetate + CO₂ + 2H+ + 2e¯ Succinate + CO₂ + 2H+ + 2e- Acetate + 3H+ + 2e¯ Ferredoxin (oxidized) + e 2H+ + 2e a-Ketoglutarate + CO₂ + 2H+ + 2e¯ Acetoacetate + 2H+ + 2e™ Pyruvate + CO₂ + H+ + 2e™ NAD+ + H+ + 2e™ NADP+ + H+ + 2e Lipoate (oxidized) + 2H+ + 2e- 1,3-Bisphosphoglycerate + 2H+ + 2e™ Glutathione (oxidized) + 2H+ + 2e FAD (free coenzyme) +2H+ + 2e™ Acetaldehyde + 2H+ + 2e Pyruvate + 2H+ + 2e Oxaloacetate + 2H+ + 2e O₂ + e a-Ketoglutarate + NH + 2H+ + 2e FAD (enzyme-bound) + 2H+ + 2e™ Methylene blue (oxidized) + 2H+ + 2e Fumarate + 2H+ + 2e¯ Q + 2H+ + 2e™ Dehydroascorbate + 2H+ + 2e Cytochrome b (+3) + e Cytochrome c₁ (+3) + e Cytochrome c (+3) + e Cytochrome a (+3) + e 0₂ + 2H+ + 2e™ Ferricyanide + 2e™ NO3 (Nitrate) + 2H+ + 2e Cytochrome ag(+3) + e Fe (+3) + e ¹0₂ + 2H+ + 2e = Reductant Pyruvate + H₂O a-ketoglutarate + H₂O Acetaldehyde + H₂O Ferredoxin (reduced) H₂ Isocitrate B-Hydroxybutyrate Malate NADH NADPH Lipoate (reduced) Glyceraldehyde-3-phosphate + P₁ 2 Glutathione (reduced) FADH₂ Ethanol Lactate Malate O₂ (superoxide) Glutamate + H₂O FADH₂ (enzyme-bound) Methylene blue (reduced) Succinate QH₂ Ascorbate Cytochrome b (+2) Cytochrome c₁ (+2) Cytochrome c (+2) Cytochrome a (+2) H₂O₂ Ferrocyanide NO₂ (Nitrite) + H₂O Cytochrome a3 (+2) Fe(+2) H₂O n 2 2 2 1 2 NN 2 2 2 2 2 2 2 NN 2 2 2 2 2 1 2 2 2 2 2 2 1 1 1 1 2 2 2 1 1 2 E° (V) -0.70 -0.67 -0.60 -0.43 -0.42 -0.38 -0.35 -0.33 -0.32 -0.32 -0.29 -0.29 -0.23 -0.22 -0.20 -0.19 -0.17 -0.16 -0.14 -0 to -0.30 0.01 0.03 0.04 0.06 0.07 0.23 0.25 0.29 0.30 0.36 0.42 0.55 0.77 0.82
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (2 reviews)
To calculate the equilibrium constant K for the glutathione peroxidase reaction at 37C using the sta...View the full answer

Answered By

Pushpinder Singh
Currently, I am PhD scholar with Indian Statistical problem, working in applied statistics and real life data problems. I have done several projects in Statistics especially Time Series data analysis, Regression Techniques.
I am Master in Statistics from Indian Institute of Technology, Kanpur.
I have been teaching students for various University entrance exams and passing grades in Graduation and Post-Graduation.I have expertise in solving problems in Statistics for more than 2 years now.I am a subject expert in Statistics with Assignmentpedia.com.
4.40+
3+ Reviews
10+ Question Solved
Related Book For 

Biochemistry Concepts And Connections
ISBN: 9780134641621
2nd Edition
Authors: Dean Appling, Spencer Anthony-Cahill, Christopher Mathews
Question Posted:
Students also viewed these Sciences questions
-
Calculate the equilibrium constant for the reaction H2 2H at a temperature of 2000 K, using properties from Table A.8. Compare the result with the value listed in Table A.10.
-
Calculate the equilibrium constant for the reaction H2 2H at a temperature of 2000 K, using properties from Table A.9. Compare the result with the value listed in Table A.11.
-
At what annual interest rate, compounded annually, would $500 have to be invested for it to grow to $1,892.84 in 12 years? Question content area bottom Part 1 The annual interest rate, compounded...
-
Line A: y = 3 0.6x Line B: y = 4 x a. Graph the linear equations and data points. b. Construct tables for x, y, Ëy, e, and e 2 similar to Table 4.4 on page 151. c. Determine which line fits the...
-
Accounting standards are set in the United States in the private sector. Public hearings and written documents provide feedback during development of the standards. Opportunity is provided to those...
-
For each variable cost per unit listed below, determine the total variable cost when units produced and sold are 40, 80, and 160 units. Direct materials .......$ 35 Direct labor ......... 65 Variable...
-
Three housewives decide to go into business publishing cookbooks. They forma corporation, having been told that the corporate form of ownership carries limited liability. They decide not to purchase...
-
Beyer Company is considering the purchase of an asset for $180,000. It is expected to produce the following net cash flows. The cash flows occur evenly throughout each year. Compute the payback...
-
Question 6 1 pts "GST is 10%. An item, to be sold in a shop, is purchased for $770 (without GST), The GST to be added before sale of the item is" O $10 O $70 O $700 O $77
-
Inflammatory stimuli cause macrophages to undergo dramatic metabolic reprogramming, including a switch from oxidative phosphorylation to aerobic glycolysis. Cordes et al. (J. Biol. Chem....
-
Given the roles of NAD + /NADH in dehydrogenation reactions and NADPH/NADP + in reductions, as discussed in Chapter 11 (Section 11.4), would you expect the intracellular ratio of NAD+ to NADH to be...
-
One mole of argon is expanded polytropically, the polytropic constant being n----1.50. In the process, the gas temperature changes by T = 26 K. Find: (a) The amount of heat obtained by the gas; (b)...
-
Canterbury Convenience Stores (CCS) is a newly formed organization in Christchurch, New Zealand. It comprises 10 moderately sized convenience stores that previously operated independently of each...
-
Orchard Distributions Pte. Ltd. is a large, Singaporean-based distributor of clothing products to other companies throughout Southeast Asia. Orders are received from customers either by telephone,...
-
You are an external auditor in a firm that undertakes the audit of Canadian Life and Mutual (CLM), a large, Montreal-based financial institution. CLM relies heavily on its computer-based information...
-
You need to temporarily increase the feed rate to an existing column without flooding. Since the column is now operating at about \(90 \%\) of flooding, you must vary some operating parameter. The...
-
Consider, again, the clothing data set. Obtain the three summary plots of the sample cross-correlations for lags 1 to 21.
-
A parallel-plate capacitor consists of two flat metal plates of area A separated by a small distance d. The plates are given equal and opposite net charges q. (a) Sketch the field lines and use your...
-
What exactly is a prima facie duty? How does an ethic of prima facie duties differ from monistic and absolutist ethical theories?
-
There is a reaction in carbohydrate metabolism in which glucose-6-phosphate reacts with NADP + to give 6- phosphoglucono-d-lactone and NADPH. In this reaction, which substance is oxidized, and which...
-
A biochemical reaction transfers 60 kJ mol -1 (15 kcal mol -1 ) of energy. What general process most likely would be involved in this transfer? What cofactor (or cosubstrate) likely would be used?...
-
The following half reactions play important roles in metabolism. Which of these two is a half reaction of oxidation? Which one is a half reaction of reduction? Write the equation for the overall...
-
View Policies Current Attempt in Progress Blossom Company owns equipment that cost $ 62, 100 when purchased on January 1, 2019. It has been depreciated using the straight- line method based on...
-
An income statement for Crandall's Bookstore for the first quarter of the current year is presented below: CRANDALL'S BOOKSTORE Income Statement for the First Quarter of the Current Year Sales...
-
At December 31, 2020, Atlanta Co. has a stock portfolio with a fair value of $40,000 and cost of $33,000. If the Securities Fair Value Adjustment (Available-for-Sale) account has a beginning debit...

Study smarter with the SolutionInn App


