Identify the conjugate acids and bases in the following pairs of substances: (a) (CH3)3 NH(CH3) 3N (c)
Question:
Identify the conjugate acids and bases in the following pairs of substances: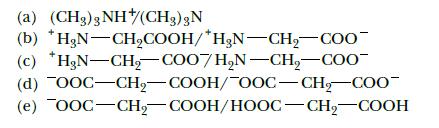
Transcribed Image Text:
(a) (CH3)3 NH(CH3) 3N (c) (b) *HgN-CH₂COOH/*H3N-CH₂-COO H₂N-CH₂-COO7H₂N-CH₂-COO (d) OOC-CH₂-COOH/OOC-CH₂-COO- (e) OOC–CH, COOH/HOOC–CH, COOH
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
a CH33NH conjugate acid CH33N conjugate base b H3N...View the full answer

Answered By

FREDRICK MUSYOKI
Professional Qualities:
Solution-oriented.
Self-motivated.
Excellent problem-solving and critical thinking skills.
Good organization, time management and prioritization.
Efficient troubleshooting abilities.
Tutoring Qualities:
I appreciate students as individuals.
I am used to tailoring resources for individual needs.
I can integrate IT into student's lessons.
I am good at explaining concepts.
I am able to help students progress.
I have a wide curriculum knowledge.
5.00+
1+ Reviews
10+ Question Solved
Related Book For 

Biochemistry
ISBN: 9781305961135
9th Edition
Authors: Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Question Posted:
Students also viewed these Sciences questions
-
Identify conjugate acids and bases in the following pairs of substances: (a) (HOCH), CNH (HOCH CNH (b) HOCH, CH, N N CH, CH, SO HOCH, CHN N CH, CH, SOs (c) O SCH, CH, N, N+CH, CH, SOg O SCH, CH,N N...
-
Identify the conjugate acids and bases in the following pairs of substances: (a) (CH 3 ) 3 NH + / (CH 3 ) 3 N (b) + H 3 NCH 2 COOH/ + H 3 NCH 2 COO - (c) + H 3 NCH 2 COO /H 2 NCH 2 COO - (d) OOCCH 2...
-
a. Consider the hydrated aluminum ion Al(H 2 O) 6 3+ as a BrnstedLowry acid. Write the chemical equation in which this ion loses a proton in a reaction with ammonia, NH 3 . Identify the conjugate...
-
Find all values of 0, if 0 is in the interval [0, 360) and has the given function value. cot 0= -1 0= (Type your answer in degrees. Use a comma to separate answers as needed.)
-
1. Suppose a UAWlabor contract with General Dynamics is being renegotiated. Some of the many issues on the table include job security, health benefits, and wages. If you are an executive in charge of...
-
A small manufacturing firm collected the following data on advertising expenditures A (in thousands of dollars) and total revenue R (in thousands of dollars). (a) Draw a scatter diagram of the data....
-
19. What is included on a budgetary comparison schedule? Is such a schedule required to be included in a CAFR?
-
A study on motivated skepticism examined whether participants were more likely to be skeptical when it served their self-interest (Ditto & Lopez, 1992). Ninety-three participants completed a...
-
Required information [The following information applies to the questions displayed below.) Sweeten Company had no jobs in progress at the beginning of March and no beginning inventories. The company...
-
Look at Figure 2.17. If you did this titration using TRIS instead of phosphate, how would the titration curve look compared to the figure? Explain. Figure 2.17 Buffering. Acid is added to the two...
-
Both RNA and DNA have negatively charged phosphate groups as part of their structure. Would you expect ions that bind to nucleic acids to be positively or negatively charged? Why?
-
An 80 kg astronaut has gone outside his space capsule to do some repair work. Unfortunately, he forgot to lock his safety tether in place, and he has drifted 5.0 m away from the capsule. Fortunately,...
-
What are the different types of drones?
-
What are the applications of drones?
-
What are the various protocols in telecom domain?
-
What are the various types of routing protocols?
-
For all the benefits they bring to business, social media and other communication technologies have created a major new challenge: responding to online rumors and attacks on a company's reputation....
-
In any hydraulic system, it is important to "bleed" air out of the line. Why?
-
Write a paper about medication error system 2016.
-
Te amino acid arginine ionizes according to the following scheme: (a) Calculate the isoelectric point of arginine. You can neglect contributions from form I. Why? (b) Calculate the average charge on...
-
It is possible to make a buï¬er that functions well near pH 7 using citric acid, which contains only carboxylate groups. Explain. Citric acid CH CO,H CH2 CO2H
-
It is possible to make a buï¬er that functions well near pH 7 using citric acid, which contains only carboxylate groups. Explain. Citric acid CH CO,H CH2 CO2H
-
firm purchased a new piece of equipment with an estimated useful life of eight years. The cost of the equipment was $65,000. The salvage value was estimated to be $10,000 at the end of year 8. Using...
-
On an average day, a company writes checks totaling $1,500. These checks take 7 days to clear. The company receives checks totaling $1,800. These checks take 4 days to clear. The cost of debt is 9%....
-
Olds Company declares Chapter 7 bankruptcy. The following are the book values of the asset and liability accounts at that time. A bankruptcy expert estimates that administrative expense will total $...

Study smarter with the SolutionInn App


