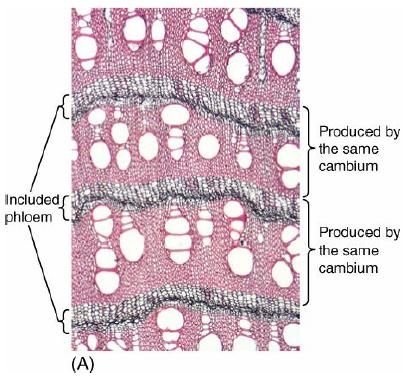
Describe the formation of included phloem. How did the included phloem of Iresine in Figure 8-30A become
Question:
Describe the formation of included phloem. How did the included phloem of Iresine in Figure 8-30A become surrounded by xylem?
Figure 8-30A
Transcribed Image Text:
Included phloem Y (Of 8.1. (A) Produced by the same cambium Produced by the same cambium
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 42% (7 reviews)
The term included phloem refers to a type of phloem tissue within a plant stem that is enclosed or surrounded by xylem tissue Phloem and xylem are two ...View the full answer

Answered By

User l_998468
I have extensive tutoring experience, having worked as a private tutor for over three years. I have tutored students from different academic levels, including high school, undergraduate, and graduate levels. My tutoring experience has taught me to be patient, attentive to student needs, and effective in communicating difficult concepts in simple terms.
I have a strong background in statistics, probability theory, data analysis, and data visualization. I am proficient in using statistical software such as R, Python, and SPSS, which are commonly used in academic research and data analysis. Additionally, I have excellent communication and interpersonal skills, which enable me to establish rapport with students, understand their learning styles, and adapt my teaching approach to meet their needs.
I am passionate about teaching and helping students achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Figure 8-3 is complex but easy to understand. Figure 8-3B shows a tree trunk, and this year is its fourth year of growth. A transverse section at the base of Figure 8-3B would show how many layers...
-
Describe the formation of hydrogen bonds in propanol, CH3CH2CH2OH. Represent possible hydrogen bonding structures in propanol by using structural formulas and the conventional notation for a hydrogen...
-
Describe the formation of hydrogen bonds in hydrogen peroxide, H2O2. Represent possible hydrogen bonding structures in hydrogen peroxide by using structural formulas and the conventional notation for...
-
I keep getting the second question wrong. Can you help me to getthat one, please thank you.I tried 8.66, and 8.67 does not are the correct answer A firm has 10 million shares outstanding with a...
-
The table gives data concerning the shrink fit of two cylinders of differing materials and dimensional specification in inches. Elastic constants for different materials may be found in Table A5....
-
If \(\$ 5,600\) is owed in taxes but there are \(\$ 4,000\) in tax credits, how much income tax is owed?
-
10. Compute January 12 2004 bid and ask volatilities (using the Black-Scholes implied volatility function) for IBM options expiring February 21. a. Do you observe a volatility smile? b. For which...
-
How has the leather and fashion group, of which LV is the anchor business, been performing? Why has LV been so successful?
-
The statement of income for Wildhorse Ltd., a private company reporting under ASPE, is presented here: WILDHORSE LTD. Statement of Income Year Ended November 30, 2021 Sales $8,200,000 Cost of goods...
-
Do roots form wood and bark, or are these secondary tissues present only in stems?
-
What is the function of lenticels? How are intercellular spaces important for this function?
-
Set up a spreadsheet similar to Figure 12-11 to solve Problem 4. FIGURE 12-11 The housing contract from Problem 3 continues on into the next year, with the last housing start occurring in April, as...
-
Locate a scholarly article relevant to how to present your financial plan for opening a Roller Skating Rink (from your draft business plan) to a lending institution--and describe your strategy for...
-
How would you expect seasonal fluctuations in demand to affect a rental company's decisions about pricing rented products such as wedding dresses or convertible cars? In terms of pricing principles,...
-
Do we drive technology, or does technology drive us? If technology drives us, what are the risks? The other side of the coin would be that we are able to stay ahead of technological transformations....
-
How do you explain the differences between the two analyses and what are the implications of using the BCG matrix in practice?
-
How do leadership styles, such as transformational leadership, shared leadership, and servant leadership, impact team dynamics, member motivation, and overall team effectiveness ?
-
An astronaut travels to a distant star with a speed of 0.75c relative to Earth. From the astronaut's point of view, the star is 7.5 ly from Earth. On the return trip, the astronaut travels with a...
-
Audrey purchases a riding lawnmower using a 2-year, no-interest deferred payment plan at Lawn Depot for x dollars. There was a down payment of d dollars and a monthly payment of m dollars. Express...
-
The pK a of CH 3 NH 2 is 40, while the pK a of HCN is 9. (a) Explain this difference in acidity. (b) Can the cyanide anion (the conjugate base of HCN) be used as a base to deprotonate a terminal...
-
For a gas that obeys the equation of state V m = Rt/P + B(T) derive the result dB(T) = B(T) T d
-
What can you say about H vaporization of a liquid as the temperature approaches the critical temperature?
-
Flexible manufacturing places new demands on the management accounting information system and how performance is evaluated. In response, a company should a. institute practices that reduce switching...
-
Revenue and expense items and components of other comprehensive income can be reported in the statement of shareholders' equity using: U.S. GAAP. IFRS. Both U.S. GAAP and IFRS. Neither U.S. GAAP nor...
-
Kirk and Spock formed the Enterprise Company in 2010 as equal owners. Kirk contributed land held an investment ($50,000 basis; $100,000 FMV), and Spock contributed $100,000 cash. The land was used in...

Study smarter with the SolutionInn App


