In Exercises complete the table for the radioactive isotope. Half-life Initial Isotope (in years) 14C 5715 Quantity
Question:
In Exercises complete the table for the radioactive isotope.
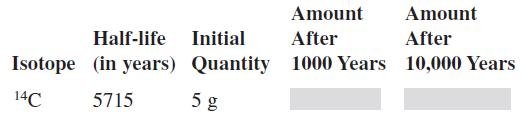
Transcribed Image Text:
Half-life Initial Isotope (in years) 14C 5715 Quantity 5 g Amount After 1000 Years Amount After 10,000 Years
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 83% (12 reviews)
Because the initial quantity is 5 grams ...View the full answer

Answered By

John Aketch
I have a 10 years tutoring experience and I have helped thousands of students to accomplish their educational endeavors globally. What interests me most is when I see my students being succeeding in their classwork. I am confident that I will bring a great change to thins organization if granted the opportunity. Thanks
5.00+
8+ Reviews
18+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
In Exercises complete the table for the radioactive isotope. Half-life Initial Isotope (in years) Quantity 226Ra 1599 20 g Amount After 1000 Years Amount After 10,000 Years
-
In Exercises complete the table for the radioactive isotope. Half-life Initial Isotope (in years) Quantity 226Ra 1599 Amount After 1000 Years 1.5 g Amount After 10,000 Years
-
In Exercises complete the table for the radioactive isotope. Isotope = 226Ra Amount Amount After Half-life Initial (in years) Quantity 1000 Years 1599 After 10,000 Years 0.1 g
-
Plastic Co Pte Ltd (Plastico) is a Fiji company. The company has issued and paid up capital of $250,000 held in equal parts by two brothers. The companys business involves making plastic products for...
-
McNamara Limiteds ledger shows the following balances on December 31, 2012: Preferred shares outstanding: 25,000 shares ...........$ 625,000 Common shares outstanding: 40,000 shares...
-
Insert the missing words: We then have to turn to the model of the system that is being evaluated. The system may be conceivedasoneof severalmodels. Evaluationwillbe basedon . . . . . . . . . . . . ....
-
Identify one organisation that you know and list a set of keywords and a phrase that you think would describe that organisation. Visit the organisations website and note the metatags used for...
-
Bay Front Co. receives $240,000 when it issues a $240,000, 10%, mortgage note payable to finance the construction of a building at December 31, 2012. The terms provide for semiannual installment...
-
Determine and briefly explain (in no more than four sentences) whether the following statements are true or false: a) It is NOT possible to construct a portfolio with zero variance of expected...
-
A and B live on adjacent plots of land. Each has two potential uses for their land, the present values of each of which depend on the use adopted by the other, as summarized in the table. All the...
-
In Exercises the general solution of the differential equation is given. Use a graphing utility to graph the particular solutions for the given values of C. 4yy' - x = 0 4y - x = C C = 0, C = 1, C =...
-
In Exercises sketch a few solutions of the differential equation on the slope field and then find the general solution analytically. dy dx -4 y -4 1
-
Consider the client buffer shown in Figure 7.3. Suppose that the streaming system uses the third option; that is, the server pllshes the media into the socket as quickly as possible. Suppose the...
-
Zephyr Minerals completed the following transactions involving machinery. Machine No. 1550 was purchased for cash on April 1, 2020, at an installed cost of $75,000. Its useful life was estimated to...
-
Kelly is a self-employed tax attorney whose practice primarily involves tax planning. During the year, she attended a three-day seminar regarding new changes to the tax law. She incurred the...
-
At a recently concluded Annual General Meeting (AGM) of a company, one of the shareholders remarked; historical financial statements are essential in corporate reporting, particularly for compliance...
-
4. In hypothesis, Mr. Ng wants to compare the solution in Q3 to other solutions in different conditions. If the following constraints are newly set in place, answer how much different is going to be...
-
3C2H6O2+7H2O= C2H4O3+11H2+O2+H2C2O4+CH2O2 Glycolic acid is produced electrochemically from ethylene glycol under alkaline conditions(NaOH). Hydrogen is produced at the cathode, and formic acid and...
-
If a circuit contains four automatic switches and we want that, with a probability of 99%, during a given time interval the switches to be all working, what probability of failure per time interval...
-
(a) Prove that form an orthonormal basis for R3 for the usual dot product. (b) Find the coordinates of v = (1, 1, 1)T relative to this basis. (c) Verify formula (5.5) in this particular case. 48-65...
-
Draw the graph of f(x) = x3 - 4x2 + 3 and its derivative f'(x) on the interval [- 2, 5] using the same axes. (a) Where on this interval is f'(x) < 0? (b) Where on this interval is f(x) decreasing as...
-
Draw the graphs of f(x) = cos x - sin (x /2) and its derivative f'(x) on the interval [0, 9] using the same axes. (a) Where on this interval is f'(x) > 0? (b) Where on this interval is f(x)...
-
In problem 1-6, find D, y using the rules of this section. 1. y = 2x2 2. y = 3x3 3. y = x 4. y = x3 5. y = 2x-2 6. y = - 3x-4
-
Arnold inc. is considering a proposal to manufacture high end protein bars used as food supplements by body builders. The project requires an upfront investment into equipment of $1.4 million. This...
-
Billy Bob bank has three assets. It has $83 million invested in consumer loans with a 3-year duration, $46 million invested in T-Bonds with a 12-year duration, and $69 million in 6-month (0.5 years)...
-
Ventaz Corp manufactures small windows for back yard sheds. Historically, its demand has ranged from 30 to 50 windows per day with an average of 4646. Alex is one of the production workers and he...

Study smarter with the SolutionInn App


