The Ideal Gas Law states that PV = nRT, where P is pressure, Vis volume, n is
Question:
The Ideal Gas Law states that PV = nRT, where P is pressure, Vis volume, n is the number of moles of gas, R is a fixed constant (the gas constant), and T' is absolute temperature. Show that
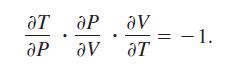
Transcribed Image Text:
aT ар ар Ꮩ . av aT - 1.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (6 reviews)
aT PV T V PV VT n RT xB PV n x...View the full answer

Answered By

JAPHETH KOGEI
Hi there. I'm here to assist you to score the highest marks on your assignments and homework. My areas of specialisation are:
Auditing, Financial Accounting, Macroeconomics, Monetary-economics, Business-administration, Advanced-accounting, Corporate Finance, Professional-accounting-ethics, Corporate governance, Financial-risk-analysis, Financial-budgeting, Corporate-social-responsibility, Statistics, Business management, logic, Critical thinking,
So, I look forward to helping you solve your academic problem.
I enjoy teaching and tutoring university and high school students. During my free time, I also read books on motivation, leadership, comedy, emotional intelligence, critical thinking, nature, human nature, innovation, persuasion, performance, negotiations, goals, power, time management, wealth, debates, sales, and finance. Additionally, I am a panellist on an FM radio program on Sunday mornings where we discuss current affairs.
I travel three times a year either to the USA, Europe and around Africa.
As a university student in the USA, I enjoyed interacting with people from different cultures and ethnic groups. Together with friends, we travelled widely in the USA and in Europe (UK, France, Denmark, Germany, Turkey, etc).
So, I look forward to tutoring you. I believe that it will be exciting to meet them.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Mathematics questions
-
The ideal gas law relates the pressure P, volume V, and temperature T of an ideal gas: PV = nRT where n is the number of moles R = 8.31145 Plots of pressure versus volume (four curves " in one plot)...
-
The ideal gas law relates the pressure P, volime V, and temperature T of an ideal gas: PV= nRT where " is the number of moles and R = 8.3145 J/(K. mol). Plots of pressure versus volume at constant...
-
The Ideal Gas Law is PV = nRT, where "n" is the number of moles and "R= 8.314 J/mol.K" is the Universal Gas Constant. 1. Define P, V, and T in the equation above and give the unit for each term in...
-
(1.71, 2.05) Use the confidence interval to find the margin of error and the sample mean.
-
Identify the animals most likely to carry rabies in the United States. Why is rabies so rare in humans and domesticated animals in developed countries?
-
please solve Based on economists' forecasts and analysis, 1-year Treasury bill rates and liquidity premiums for the next four years are expected to be as follows: RU (21) E() - (1) - 1.60% 2.sex...
-
(Two or more contracts) Construction Limited is engaged on two contracts A and B during the year. The following particulars are obtained at the end of December 1998. Contracts A April 1 Rs B...
-
My firm has a wage contract with the union. Therefore, we do not need to compute a labor price variance; it will always be zero. Comment.
-
What is one thing that you enjoyed for tolerated) in your Financial Accounting course? What is one thing that you did NOT enjoy in your Financial Accounting course
-
A pharmaceutical corporation has two plants that produce the same over-the-counter medicine. If x 1 and x 2 are the numbers of units produced at plant 1 and plant 2, respectively, then the total...
-
The utility function U = (x, y) is a measure of the utility (or satisfaction) derived by a person from the consumption of two products x and y. The utility function for two products is (a) Determine...
-
Explain the relationship between the demand elasticity and the excess capacity that occurs for a monopolistic competitor.
-
Problem 8-19 (Algo) Cash Budget; Income Statement; Balance Sheet [LO8-2, LO8-4, LO8-8, LO8-9, LO8- 10] Minden Company is a wholesale distributor of premium European chocolates. The company's balance...
-
Consider the unsteady flow of a fluid in the x direction through a control volume. The linear momentum of the fluid within the control volume is a function of time given by 200ti slug*ft/s, where t...
-
For a continuous uniform distribution with u = 0 and o = 1, the minimum is - V3 and the maximum is V3. For this continuous uniform distribution, find the probability of randomly selecting a value...
-
Marc Goudreau, administrator of Clearwater Hospital, was puzzled by the prior month's reports. "Every month, it's anyone's guess whether the lab will show a profit or a loss. Perhaps the only answer...
-
A system consisting of a gas consisting of O2 (32 Da), H2 (2 Da), and Ar (40 Da) molecules and a billiard ball is at some temperature . Relative to O2, the billiard ball is 1.0 E+26 times as massive...
-
An unknown charged particle passes without deflection through crossed electric and magnetic fields of strengths 187,500 V/m and 0.1250 T, respectively. The particle passes out of the electric field,...
-
What exactly is a prima facie duty? How does an ethic of prima facie duties differ from monistic and absolutist ethical theories?
-
The ________ of A with B consists of all elements in both A and B.
-
True or False. The intersection of two sets is always a subset of their union.
-
True or False. If A is a set, the complement of A is the set of all the elements in the universal set that are not in A.
-
you are analyzing the cost of debt for a firm. Do you know that the firms 14 year maturity, 7.8 Percent coupon bonds are selling at a price of $834. The Barnes pay interest semi annually. If these...
-
***Please answer the following using excel and showcasing the formulas/calculations used*** thank you so much Financial information on AAA Ltd. is shown below. AAA Ltd. Income Statement For the Year...
-
2. In an account Anh Paglinawan currently has $216,670.00. At a rate of 8.00% how long will it take for them to have $298,390.00 assuming semi-annually compounding? (Hint: compute the exact years, do...

Study smarter with the SolutionInn App


