0.10 mol of a monatomic gas follow the process shown in Figure P 17.60. a. How much...
Question:
0.10 mol of a monatomic gas follow the process shown in Figure P 17.60.
a. How much heat energy is transferred to or from the gas during process 1 → 2?
b. How much heat energy is transferred to or from the gas during process 2 → 3?
c. What is the total change in thermal energy of the gas?
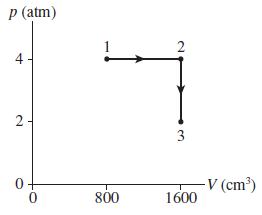
Transcribed Image Text:
р (atm) 2 4 - 3 V (cm) 800 1600
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (9 reviews)
Model The monatomic gas is an ideal gas which is subject to isobaric and isochori...View the full answer

Answered By

Mugdha Sisodiya
My self Mugdha Sisodiya from Chhattisgarh India. I have completed my Bachelors degree in 2015 and My Master in Commerce degree in 2016. I am having expertise in Management, Cost and Finance Accounts. Further I have completed my Chartered Accountant and working as a Professional.
Since 2012 I am providing home tutions.
3.30+
2+ Reviews
10+ Question Solved
Related Book For 

Physics For Scientists And Engineers A Strategic Approach With Modern Physics
ISBN: 9780321740908
3rd Edition
Authors: Randall D. Knight
Question Posted:
Students also viewed these Mathematics questions
-
A heat engine using 1.0 mol of a monatomic gas follows the cycle shown in FIGURE P21.55. 3750 J of heat energy is transferred to the gas during process 1 2. a. Determine W s , Q, and ÎE th for...
-
A gas undergoes the process shown in FIGURE Q18.10. By what factor does the temperature change? FIGURE Q18.10
-
Heat is added isothermally to 2.5 mol of a monatomic ideal gas. The temperature of the gas is 430 K. How much heat must be added to make the volume of the gas double?
-
Single PlantwideandMultiple Production Department Factory Overhead Rate Methodsand Product Cost Distortion The management of Nova Industries Inc. manufactures gasoline and diesel engines through two...
-
A gas refrigeration system using air as the working fluid has a pressure ratio of 5. Air enters the compressor at 0oC. The high-pressure air is cooled to 35oC by rejecting heat to the surroundings....
-
Abe's Steakhouse is the largest upscale steakhouse company in the United States, based on total company- and franchisee-owned restaurants. The company's menu features a broad selection of...
-
Why is it important to evaluate your change management experiences and document them as lessons learned? AppendixLO1
-
Mike and Linda have three dependent children who are full-time students in 2015. Mike and Lindas taxable income is $180,000 and they provided $8,000 of support for each child. Information for each...
-
You are considering the following two mutually exclusive projects. The crossover rate between these two projects is ___ percent and Project ___ should be accepted if the required return is greater...
-
Pin B has a mass m and slides along the slot in the rotating arm OC and along the slot DE which is cut in a fixed horizontal plate. Neglecting friction and knowing that rod OC rotates at the constant...
-
Figure P17.57 shows two processes that take a gas from state i to state f. Show that Q A Q B = p i V i . 2p Pi- 0+ V, 2V,
-
a. What compression ratio V max /V min will raise the air temperature from 20 C to 1000 C in an adiabatic process? b. What pressure ratio p max /p min does this process have?
-
Understand how planning as a personal process can help shape your career.
-
3. Suppose we have n i.i.d., uniform-(0,t) random variables. Place these random variables on the interval (0, t]. Let 0 = 80 < 81 < ... < Sn1 < (0,t]. Skt. Compute the probability that there are in...
-
3. (3 pts) Use Python to write a function that takes a single input, a list of numbers. The function should loop through the list and, on each iteration, print the number if it is the largest number...
-
a) A linear charge density = 4z C/m is distributed on the z axis, what is the total charge within a cylinder of radius r = 0.5 m and height h = 5 m which extends from z = 1 to z = 4? b) A uniform...
-
Read the articles given below on module 9 now read the articles given below on module 10 Now answer these questions based on both modules slideshow pictures and the links readings Describe how the...
-
2. For each equation, state the quantity (with units) represented by each variable. a) D=mV F b) P== A c) P = Dgh g 3. Write a single sentence answering each question. a) If the mass remains constant...
-
Find the inverse of 1 1 2 C = 1 1 0 -1 -1 -2 3.5 -2 -2.5 -0.5 2 2.5 -2 1.5 -1.5 1 -1 -3 3.
-
A report from the college dean indicates that for the previous semester, the grade distribution for the Department of Psychology included 135 As, 158 Bs, 140 Cs, 94 Ds, and 53 Fs. Determine what kind...
-
Bob, who has a mass of 75 kg, can throw a 500 g rock with a speed of 30 m/s. The distance through which his hand moves as he accelerates the rock from rest until he releases it is 1.0 m. a. What...
-
Two packages at UPS start sliding down the 20° ramp shown in FIGURE P7.33. Package A has a mass of 5.0 kg and a coefficient of friction of 0.20. Package B has a mass of 10 kg and a coefficient of...
-
Two packages at UPS start sliding down the 20° ramp shown in FIGURE P7.33. Package A has a mass of 5.0 kg and a coefficient of friction of 0.20. Package B has a mass of 10 kg and a coefficient of...
-
Slow Roll Drum Co. is evaluating the extension of credit to a new group of customers. Although these customers will provide $198,000 in additional credit sales, 13 percent are likely to be...
-
Wendell's Donut Shoppe is investigating the purchase of a new $39,600 conut-making machine. The new machine would permit the company to reduce the amount of part-time help needed, at a cost savings...
-
1.Discuss the challenges faced with Valuing Stocks and Bonds. As part of this discussion, how will the selected item be implemented in an organization and its significance? 2. Discuss how Valuing...

Study smarter with the SolutionInn App


