Question: A heat engine using 1.0 mol of a monatomic gas follows the cycle shown in FIGURE P21.55. 3750 J of heat energy is transferred to
A heat engine using 1.0 mol of a monatomic gas follows the cycle shown in FIGURE P21.55. 3750 J of heat energy is transferred to the gas during process 1 †’ 2.
a. Determine Ws, Q, and ΔEthfor each of the four processes in this cycle. Display your results in a table.
b. What is the thermal efficiency of this heat engine?
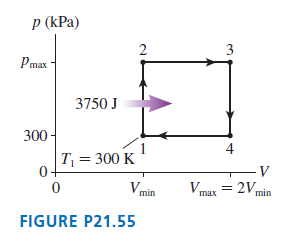
p (kPa) 3 Pmax 3750 J 300 - T = 300 K 0- Vmin Vmax = 2V, min FIGURE P21.55 4)
Step by Step Solution
3.47 Rating (173 Votes )
There are 3 Steps involved in it
Model The heat engine follows a closed cycle with process 1 2 and process 3 4 being isochoric and pr... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
1442_6054778b9c7bc_693675.pdf
180 KBs PDF File
1442_6054778b9c7bc_693675.docx
120 KBs Word File


