Study the following scheme. a. Give the names and formulae of substances A to E. b. Describe
Question:
Study the following scheme.
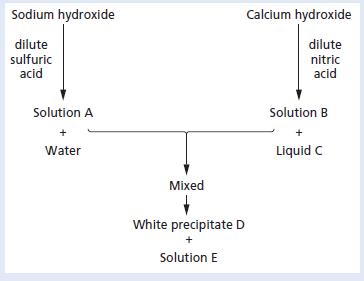
a. Give the names and formulae of substances A to E.
b. Describe a test which could be used to identify the presence of water.
c. Which indicator is suitable for the initial reaction between the hydroxides and the dilute acids shown?
d. Write balanced chemical equations for the reactions taking place in the scheme.
e. Write an ionic equation for the production of the white precipitate D.
Sodium hydroxide Calcium hydroxide dilute sulfuric acid dilute nitric acid Solution A Solution B Water Liquid C Mixed White precipitate D Solution E
Step by Step Answer:
a Substance A Sodium Hydroxide NaOH Substance B Sulphuric Acid H2SO4 Substance C Pot...View the full answer



Related Video
The experiment aims to show the impact of various beverages on teeth by using eggs as a representation of enamel. Three eggs are boiled and then placed in glasses filled with fizzy drinks, vinegar, and mango juice for 24 hours. The shells of eggs are similar to enamel as they are composed of calcium carbonate, and enamel is primarily made of calcium phosphate. The eggs are then observed to demonstrate the effects of the different liquids on teeth and the importance of brushing regularly. The egg placed in fizzy drink has turned dark in color but can be cleaned by brushing with toothpaste and rinsing with water. The egg placed in vinegar has had its shell softened due to the chemical reaction of vinegar and calcium carbonate, which can\'t be reversed. This highlights the fact that acids are more damaging to teeth than other substances. The egg placed in mango juice represents the process of bacteria in the mouth converting sugars and starches into acids that form plaque, which can be prevented by brushing. The use of fluoride in toothpaste is also highlighted as it slows down the demineralization process and protects the enamel. The importance of brushing teeth twice a day is emphasized.
Students also viewed these Sciences questions
-
The equation for the initial reaction between an alcohol (primary or secondary) and iodine solution in the presence of aqueous sodium hydroxide is: RCH(OH)R'() + I2(aq) + 2NaOH(aq) RCOR'(aq) +...
-
Write balanced chemical equations for each of the following reactions: (a) The nitric oxide molecule undergoes photodissociation in the upper atmosphere. (b) The nitric oxide molecule undergoes...
-
a. Give the names and formulae of the two major greenhouse gases. b. Name a natural source of the gases you have named in part a. c. Name a man-made source of the gases you have named in part a.
-
This is a stocklist case arising under 220(b) of our [Delaware] General Corporation Law. The issue is whether a shareholder states a proper purpose for inspection under our statute in seeking to...
-
A wine was tested for acidity, and its pH was found to be 3.85 at 25C. What is the hydronium-ion concentration?
-
Refer to your answers from E17A-6. Requirements 1. Prepare the journal entries to record the assignment of direct materials and direct labor, and the allocation of manufacturing overhead to the...
-
5. (a) Let I be a bounded interval. Prove that if f : I -t R is uniformly continuous on I, then f is bounded on I. (b) Prove that (a) may be false if I is unbounded or if f is merely continuous
-
Sanville Quarries is considering acquiring a new drilling machine that is expected to be more efficient than the current machine. The project is to be evaluated over four years. The initial outlay...
-
Grace Carol Associates surveys American eating habits. The company's accounts include Land, Buildings, Office Equipment, and Communication Equipment, with a separate Accumulated Depreciation account...
-
Calculate the NPV of the proposed overhaul of the Vital Spark, with and without the new engine and control system. To do the calculation, you will have to prepare a spreadsheet table showing all...
-
In a titration involving 24.0 cm 3 potassium hydroxide solution against a solution containing 1 mol dm 3 of sulfuric acid, 28.0 cm 3 of the acid was found to just neutralise the alkali completely. a....
-
a. Copy out and complete the table, which covers the different methods of preparing salts. b. Write word and balanced chemical equations for each reaction shown in your table. Also write ionic...
-
How many known planets are in our solar system?
-
My first run at a dissertation was on Dr. Martin Luther King, Jr. When I was very young he walked through my hometown of Albany, Georgia. My father accompanied him, more to protect him than anything,...
-
Question 2 are charged, and the charge on sphere Y is The X and Y dots shown in the figure are two identical spheres, X and Y, that are fixed in place with their centers in the plane of the page....
-
how do i get the residuel income please help in just need the cell formula in excel 2 Genmure Corporation is trying to analyze the results of three efficiency initiatives that were taken on the...
-
Harlow Appliance has just developed a new air fryer it believes will have broad market appeal. The company has performed marketing and cost studies that revealed the below information: a. New...
-
Based on the business that you created a global strategy for in the week 4 discussion, determine a low-cost & differentiation strategy in an effort to remain competitive in the global market. Include...
-
At December 31, 2020, Redmond Company has outstanding three long-term debt issues. The first is a $2,000,000 note payable which matures June 30, 2023. The second is a $6,000,000 bond issue which...
-
Respond to the ethical judgments required based on the following scenarios. Scenario 1. Assume you have collected a sample using MUS and that you have evaluated that sample to calculate a total...
-
A gas obeys the equation of state Vm = RT/p + aT2 and its constant pressure heat capacity is given by Cp,m = A + BT + Cp, where a, A, B, and Care constants independent of T and p. Obtain expressions...
-
What fraction of the enthalpy of vaporization of ethanol is spent on expanding its vapour?
-
There are no dietary recommendations for consumption of carbohydrates. Some nutritionists recommend diets that are largely devoid of carbohydrates, with most of the energy needs being met by fats....
-
Jeannie is an adjunct faculty at a local college, where she earned $680.00 during the most recent semimonthly pay period. Her prior year-to-date pay is $18,540. She is single and has one withholding...
-
The company sold merchandise to a customer on March 31, 2020, for $100,000. The customer paid with a promissory note that has a term of 18 months and an annual interest rate of 9%. The companys...
-
imer 2 0 2 4 Question 8 , PF 8 - 3 5 A ( similar to ) HW Score: 0 % , 0 of 1 0 0 points lework CH 8 Part 1 of 6 Points: 0 of 1 5 Save The comparative financial statements of Highland Cosmetic Supply...

Study smarter with the SolutionInn App


