a. Copy out and complete the table, which covers the different methods of preparing salts. b. Write
Question:
a. Copy out and complete the table, which covers the different methods of preparing salts.
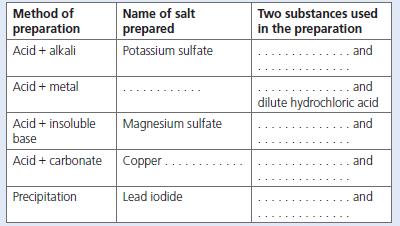
b. Write word and balanced chemical equations for each reaction shown in your table. Also write ionic equations where appropriate.
Name of salt prepared Two substances used in the preparation Method of preparation Acid + alkali Potassium sulfate and Acid + metal .and dilute hydrochloric acid Acid + insoluble base Magnesium sulfate and Acid + carbonate Copper. and Precipitation Lead iodide and
Step by Step Answer:



Related Video
The experiment aims to show the impact of various beverages on teeth by using eggs as a representation of enamel. Three eggs are boiled and then placed in glasses filled with fizzy drinks, vinegar, and mango juice for 24 hours. The shells of eggs are similar to enamel as they are composed of calcium carbonate, and enamel is primarily made of calcium phosphate. The eggs are then observed to demonstrate the effects of the different liquids on teeth and the importance of brushing regularly. The egg placed in fizzy drink has turned dark in color but can be cleaned by brushing with toothpaste and rinsing with water. The egg placed in vinegar has had its shell softened due to the chemical reaction of vinegar and calcium carbonate, which can\'t be reversed. This highlights the fact that acids are more damaging to teeth than other substances. The egg placed in mango juice represents the process of bacteria in the mouth converting sugars and starches into acids that form plaque, which can be prevented by brushing. The use of fluoride in toothpaste is also highlighted as it slows down the demineralization process and protects the enamel. The importance of brushing teeth twice a day is emphasized.
Students also viewed these Sciences questions
-
Write the balanced chemical equations for (a) The complete combustion of acetic acid (CH3COOH), the main active ingredient in vinegar (b) The decomposition of solid calcium hydroxide into solid...
-
For each reaction shown here, identify the nucleophile, its atom, the eletrophilic atom in the substate molecule, and the leaving groups. Write the organic product of the reaction. Br + NaSH (a) CHl...
-
Write the balanced molecular and net ionic equations for the reaction that occurs when the contents of the two beakers are added together. What colors represent the spectator ions in each reaction?...
-
Which of the following expressions correctly returns an integer that represents the month of a Local Date object named hireDate? a. GetMonth(hireDate) b. GetMonthValue(hireDate) c....
-
The pH of a cup of coffee (at 25C) was found to be 5.12. What is the hydronium-ion concentration?
-
Samson Winery in Pleasant Valley, New York, has two departments: Fermenting and Packaging. Direct materials are added at the beginning of the fermenting process (grapes) and at the end of the...
-
4. (a) Suppose that f: [0,00) -t R is continuous and there is an L E R such that f(x) -t L as x -t 00. Prove that f is uniformly continuous on [0,00). (b) Prove that f (x) = 1/ (x2 + 1) is uniformly...
-
A boarding stable feeds and houses work horses used to pull tourist-filled carriages through the streets of a historic city. The stable owner wishes to strike a balance between a healthy nutritional...
-
What is bond rating? What is its importance? Give an example of bond rating. What is the purpose of computing the equivalent taxable yield of a municipal bond? Explain why high income and wealthy...
-
a. Construct a frequency distribution table for ages of female participants using the classes 2029, 3039, 4049, 5059, and 6069. b. Calculate the relative frequency and percentage for each class. c....
-
Study the following scheme. a. Give the names and formulae of substances A to E. b. Describe a test which could be used to identify the presence of water. c. Which indicator is suitable for the...
-
Explain, with the aid of examples, what you understand by the following terms: a. Strong acid b. Weak acid c. Strong alkali d. Weak alkali e. Concentrated acid.
-
Information gathering and analysis: Does KCI have a potential loss contingency? Discuss procedures the auditors should perform to gather evidence about this situation. King Companies, Inc. (KCI) is a...
-
Joint Ventures are a common Mode of Entry in international business. Appreciate if in-depth elaboration provided on its advantages and disadvantages. Also briefly mention the factors which make joint...
-
The field excursion is intended to give students an opportunity to carry out an applied geographical research project based on observation, data recording, and analysis. Using a field site of your...
-
An angry coworker is expressing their needs through a rush of emotion and snide comments while another coworker is trying to interpret them to provide some help and support. You are a manager and...
-
You may have a general understanding of the difference between ethics and legality , but could you explain the distinction? It is not always easy to know where to draw the line between the two. Some...
-
Someone can be a good leader but not be a very good manager and vice-versa. Leadership is creating a vision for others to follow, establishing corporate values and ethics, and transforming the way...
-
Strickland Company owes $200,000 plus $18,000 of accrued interest to Moran State Bank. The debt is a 10-year, 10% note. During 2020, Stricklands business deteriorated due to a faltering regional...
-
TRUE-FALSE QUESTIONS 1. In terms of preliminary analytical procedures, assume that the company has introduced a new product with a low price point and significant customer demand. The auditor would...
-
The temperature dependence of the vapour pressure of solid sulfur dioxide can be approximately represented by the relation log (p/Torr) = 10.5916 - 1871.2/ (T/K) and that of liquid sulfur dioxide by...
-
The temperature dependence of the vapour pressure of solid sulfur dioxide can be approximately represented by the relation log (p/Torr) = 10.5916 - 1871.2/ (T/K) and that of liquid sulfur dioxide by...
-
Glucose and fructose are simple sugars with the molecular formula C6HI206 Sucrose, or table sugar, is a complex sugar with molecular formula C12H220I1 that consists of a glucose unit covalently bound...
-
As a Financial Analyst in the Finance Department of Zeta Auto Corporation they are seeking to expand production. The CFO asks you to help decide whether the firm should set up a new plant to...
-
Chapter 4 When an Auditor finds misstatements in entities financial statements which may be the result of fraudulent act, what should be the role of an auditor under that situation? (2 Points)
-
Suppose the following input prices are provided for each year: Required: $

Study smarter with the SolutionInn App


