a. Copy the waves shown in the diagram onto a sheet of graph paper and use the
Question:
a. Copy the waves shown in the diagram onto a sheet of graph paper and use the principle of superposition to sketch the resultant wave.
b. Compare the wavelength of the resultant wave with that of the component waves.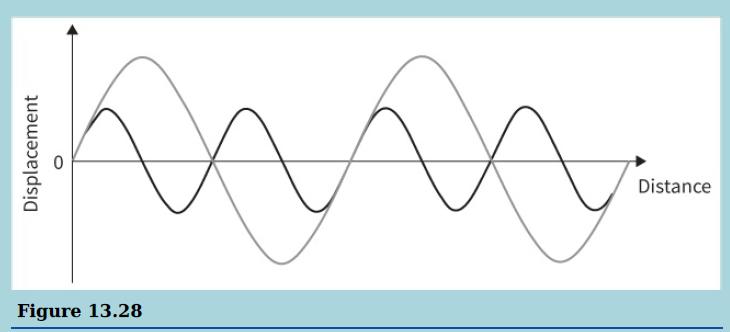
Transcribed Image Text:
Distance Figure 13.28
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 76% (13 reviews)
a To find the resultant wave using the principle of superposition we add the amplitude of the compon...View the full answer

Answered By

Akshay Shete
I have extensive experience as a tutor, both online and in-person. I have worked with students of all ages and abilities, and am skilled at adapting my teaching style to meet the needs of each individual student. I have a strong background in a variety of subjects, including math, science, and English, and am able to break down complex concepts in a way that is easy for students to understand. In addition to my subject matter expertise, I am also a patient and supportive teacher, and am committed to helping my students succeed. Whether I am working with a struggling student who needs extra help to catch up, or an advanced student looking to get ahead, I am able to provide the guidance and support they need to reach their goals. Overall, my hands-on experience as a tutor has prepared me to be a confident and effective teacher, and I am excited to use my skills to help students succeed.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:
Students also viewed these Sciences questions
-
Use graph paper and sketch the phase diagram of oxygen, O2, from the following information: normal melting point, 218C; normal boiling point, 183C; triple point, 219C, 1.10 mmHg; critical point,...
-
Use graph paper and sketch the phase diagram of argon, Ar, from the following information: normal melting point, 187C; normal boiling point, 186C; triple point, 189C, 0.68 atm; critical point, 122C,...
-
Use the superposition principle to obtain vx in the circuit of Fig. 10.89. Let vs = 50sin 2t V and is = 12cos(6t +10°) s A. 2002 5H i, (+) 16
-
A ball, which we can treat as a point charge, has a charge of +Q. This ball is 50 cm away from a ball of charge-100, which is fixed in position. The +Q ball is 30 cm vertically below, and 40 cm...
-
A debt of $60 000 is repaid over 25 years with monthly payments. Interest is 7% compounded semi-annually. (a) What is the size of the periodic payments? (b) What is the outstanding principal after...
-
1. What evidence of questionnaires have you found at MRE? Be specific about what you have found and where. 2. Critique the questionnaire that Snowden circulated. What can be done to improve its...
-
9 BMW introdujo hace poco su primer vehculo deportivo utilitario, el X6, para competir con otros populares vehculos 44, como el Mercedes-Benz R-class. Disee un programa de marketing directo para...
-
Air at a flow rate of 12,000 scfm (60?F, 1 atm) and containing 0.5 mol% ethyl acetate (EA) and no water vapor is to be treated with activated carbon (C) (?b = 30 lb/ft3) with an equivalent particle...
-
The Golden Mushroom has two classes of stock authorized: 8 % , $ 1 0 par preferred, and $ 1 par value common. The following transactions affect stockholders' equity during 2 0 2 4 , its first year of...
-
Techno, a unit of Outdoor Corporation, manufactures a line of electric, cordless, lawn mowers. Senior management of Outdoor Corporation has noticed that Techno has been producing more lawn mowers...
-
State how the diffracted pattern will change when: a. The wavelength of the incident wave is increased b. The wavelength of the incident wave is decreased.
-
A microwave oven (Figure 13.9) uses microwaves with a wavelength of 12.5 cm. The front door of the oven is made of glass with a metal grid inside; the gaps in the grid are a few millimetres across....
-
What are (a) the heat \(Q_{\mathrm{H}}\) extracted from the hot reservoir and (b) the efficiency for a heat engine described by the \(p V\) diagram of Figure P12.104? Figure P12.104 p (kPa) 300- 200-...
-
1. List at least three (3) ways a business can anticipate potential problems to prevent complaints. 2. Explain how to identify customer needs and expectations. 3. List at least five (5) ways to build...
-
you have taken over a company with 4 employees and you have 1 millon dollars with you.as business management student,you are expected to take 5 business decisions ensuring that the company is able to...
-
Draw the Diamond - E ( SERVO ) model of strategic management and provide ONE WORD ( or short phrase ) that best describes the relationships between the elements of the model. ( up to 1 0 points )
-
If a patient's X-ray is rejected (at the end of the 22-minute evaluation by the doctor), she has a second X-ray taken (assume that the second X-ray will always be accepted) and this new X-ray must be...
-
What are the cognitive appraisal processes involved in stress perception, and how can cognitive-behavioral techniques such as cognitive restructuring and mindfulness-based interventions help...
-
Pebble Securities is a corporation that buys and sells financial assets. It purchases notes receivable from manufacturers that need cash immediately and cannot wait to collect the notes. Pebble pays...
-
What is the order p of a B + -tree? Describe the structure of both internal and leaf nodes of a B + -tree.
-
Explain why attractive interactions between molecules in gas make the pressure less than that predicted by the ideal gas equation of state.
-
Draw resonance structures for each of the following radicals: (a) (b) (c) (d)
-
Calculate the pressure exerted by benzene for a molar volume of 2.00 L at 595 K using the RedlichKwong equation of state: The RedlichKwong parameters a and b for benzene are 452.0 bar dm 6 mol 2 K...
-
During the month of September,the Cider Pressing Company is trying to determine how much cider they are going to sell in October and November. One gallon of cider typically sells for $7 per gallon....
-
This is very confusing please help with descriptions if possible. Complete this question by entering your answers in the tabs below. Prepare a master budget for the three-month period ending June 30...
-
Doug recibe un dplex como regalo de su to. La base del to para el dplex y el terreno es de $90,000. En el momento de la donacin, el terreno y el edificio tienen un FMV de $40 000 y $80 000,...

Study smarter with the SolutionInn App


