This diagram shows the electrolysis of copper chloride. a i. On a copy of the diagram, mark
Question:
This diagram shows the electrolysis of copper chloride.
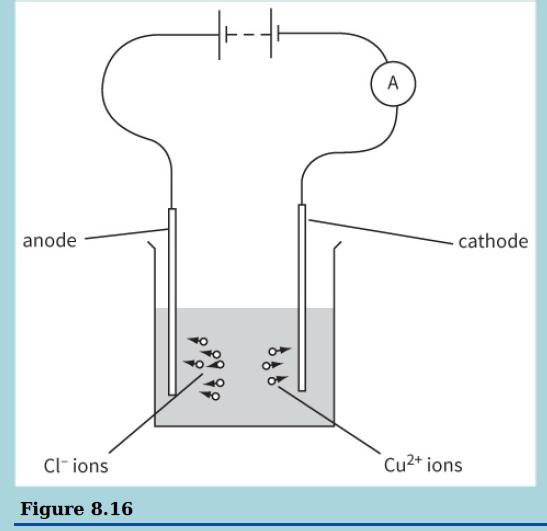
a i. On a copy of the diagram, mark the direction of the conventional current in the electrolyte. Label it conventional current.
ii. Mark the direction of the electron flow in the connecting wires. Label this electron flow.
b. In a time period of 8 minutes, 3.6 × 1016 chloride (Cl−) ions are neutralised and liberated at the anode and 1.8 × 1016 copper (Cu2+) ions are neutralised and deposited on the cathode.
i. Calculate the total charge passing through the electrolyte in this time.
ii. Calculate the current in the circuit.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Physics Coursebook
ISBN: 9781108859035
3rd Edition
Authors: David Sang, Graham Jones, Gurinder Chadha, Richard Woodside
Question Posted:





