A cell with a water potential of 300 kPa was placed in pure water at time zero.
Question:
A cell with a water potential of –300 kPa was placed in pure water at time zero. The rate of entry of water into the cell was measured as the change in water potential with time. The graph shows the results of this investigation.
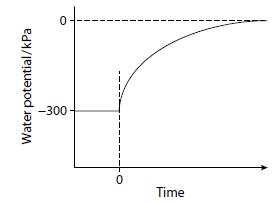
Describe and explain the results obtained.
Transcribed Image Text:
-300 Time Water potential/kPa
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (12 reviews)
Water potential of pure water is zero Water potential of water decreases as solutes are added As mor...View the full answer

Answered By

Keerthika K
I have been the topper of my class in undergraduate and postgraduate studies. I also have immense online tutoring experience where I help students in understanding their concepts in a crystal clear manner. I am also strongly analytical which helps me answer every question that requires critical thinking.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Biology
ISBN: 9781107636828
4th Edition
Authors: Mary Jones, Richard Fosbery, Jennifer Gregory, Dennis Taylor
Question Posted:
Students also viewed these Sciences questions
-
At time zero the price of a non-dividend-paying stock is S 0 . Suppose that the time interval between 0 and T is divided into two subintervals of length t 1 and t 2 . During the first subinterval,...
-
Water initially at 300 kPa and 0.5 m3/kg is contained in a piston-cylinder device fitted with stops so that the water supports the weight of the piston and the force of the atmosphere. The water is...
-
A mass of 5 kg of saturated water vapor at 300 kPa is heated at constant pressure until the temperature reaches 200C. Calculate the work done by the steam during this process.
-
How many objects are eligible for garbage collection at the end of the main() method? A. None. B. One. C. Two. D. Three. E. The code does not compile. F. None of the above. package store; public...
-
The blue line of the strontium atom emission has a wavelength of 461 nm. What is the frequency of this light? What is the energy of a photon of this light?
-
A two dimensional quadrupole consisting of four charges +q, -q, +q and -q located at the points (a2,0),(-a2,0),(0,a2) and (0.-a2). calculate the quadrupole moment tensor [12ga (a) -12ga o [1qa -12ga...
-
Great Lakes Metals, Inc., has two divisions. The fabrication division transfers partially completed components to the assembly division at a predetermined transfer price. The fabrication division,...
-
You are performing an information system audit to evaluate internal controls in Aardvark Wholesalers (AW) computer system. From an AW manual, you have obtained the following job descriptions for key...
-
Please provide a summary and analysis of the article: Retroactive Taxation, Unfunded Pensions, and Shadow Bankruptcies by Julie A. Roin
-
The capital allocation process involves the transfer of capital among different entities that include individuals, small businesses, banks, financial intermediaries, companies, mutual funds, and...
-
Copy and complete the table below to compare cell walls with cell membranes. Feature Cell wall Cell membrane is the thickness normally measured in nm or um? location chemical composition permeability...
-
The rate of movement of molecules or ions across a cell surface membrane is affected by the relative concentrations of the molecules or ions on either side of the membrane. The graphs below show the...
-
Key comparative figures for both Best Buy and Circuit City follow. Required 1. Compute sales-per-employee for 2005 and 2004 for each company. 2. Comment on each companys operating efficiency based on...
-
Problem 2.01 An ant is crawling along a straight wire, which we shall call the x axis, from A to B to C to D (which overlaps A), as shown in the figure below. O is the origin. Suppose you take...
-
In a separate C++ program, do the following: a) Create an unordered linked list by declaring a linked list of the unordered LinkedList type. You may assume that this list is to be comprised of...
-
TranscribedText: El. You are sitting at a table that has a solid round top {5.1"} kg] and a single solid cvlindrical leg {4. kg) [see figure, note that the tilt angle is exaggerated to he...
-
The municipal mill rate in the neighbourhood is 22.375 mills. There is an educational mill rate of 11.35 mills. The following list is the municipalities planned local improvement costs for the next...
-
Maggie Company had the following functional income statement for the month of May, 2020: MAGGIE COMPANY Functional Income Statement For the Month Ending May 31, 2020 Sales (30,000 units) $300,000...
-
If Kerry Dahl invests $3,152 now, she will receive $10,000 at the end of 15 years. What annual rate of interest will Kerry earn on her investment? Round your answer to the nearest whole number. Show...
-
Time Solutions, Inc. is an employment services firm that places both temporary and permanent workers with a variety of clients. Temporary placements account for 70% of Time Solutions' revenue;...
-
Identify the reagents you would use to convert 2-bromo-2-methylbutane into 3-methyl-1-butyne.
-
Would ethanol (CH 3 CH 2 OH) be a suitable solvent in which to perform the following proton transfer? Explain your answer: ONH2 + NH3 NH2 H.
-
Identify the reagents you would use to convert 1-pentene into a geminal dibromide (geminal indicates that both bromine atoms are connected to the same carbon atom).
-
Comparative financial statements for Weller Corporation, a merchandising company, for the year ending December 31 appear below. The company did not issue any new common stock during the year. A total...
-
Mrquered Mrquered
-
You plan to invest $10,00 today in an investment account earning 5% interest. You then plan to invest an additional $1,000 into this account each year for the next twenty years. How much money will...

Study smarter with the SolutionInn App


