a. The table shows the first five ionisation energies for five elements (A to E). For each
Question:
a. The table shows the first five ionisation energies for five elements (A to E). For each one state which group the element belongs to.
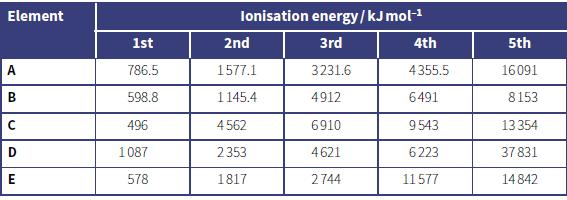
b. Explain your reasoning behind your answer for element E.
c. Draw a sketch graph to show how log10 ionisation energy for phosphorus (atomic number 15) varies when plotted against number of electrons removed.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





