Predict the major product(s) for each of the following reactions: 1) MCPBA 1) , THF 2),, NaOH
Question:
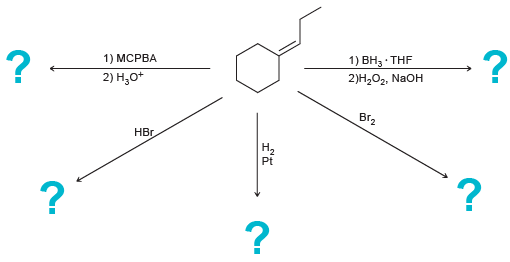
Transcribed Image Text:
1) MCPBA 1) ВН, THF 2)Н,О, NaOH 2) Н,о* ВГz НЕ. На Pt
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 70% (20 reviews)
HO OH En Br 1 M...View the full answer

Answered By

Hamza Amjad
Currently I am student in master degree program.from last two year I am tutring in Academy and I tought many O/A level student in home tution.
4.80+
3+ Reviews
10+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Sciences questions
-
Predict the major product (or products) formed when each of the following reacts with a mixture of concentrated HNO3 and H2SO4. (a) (b) (c) 4-Chlorobenzoic acid (d) 3-Chlorobenzoic acid (e)...
-
Predict the major product from each of the following reactions. (a) (b) (c) (d) (e) (f) (g) (h) (i) CH NH2, cat. HA N-H, cat. HA NH2 cat. HA PPha (1) HS SH (2) Raney Ni, H2 0 CH2PPha (excess) O. O...
-
Predict the major product from each of the following reactions. (a) (b) (c) (d) (e) HO OH NH2 HA (cat.) (1) HCN (2) LiAIH4 (3) H2o mCPBA
-
Assume that 3-month Treasury bills totaling $23 billion were sold in $10,000 denominations at a discount rate of 5.200%. In addition, the Treasury Department sold 6-month bills totaling $21 billion...
-
Develop and prototype a new interface design for the system's function using Graphical User Interface. Include common interface functions such as (textboxes, radio button, drop-down menus, check...
-
The 50-lb load is hoisted by the pulley system and motor M. If the motor exerts a constant force of 30 lb on the cable, determine the power that must be supplied to the motor if the load has been...
-
Explain the classification of cost in detail.
-
Review the Comprehensive Annual Financial Report (CAFR) that you obtained. 1. What are three main sections of the report? 2. Review the introductory section of the CAFR. a. Was the entitys annual...
-
The mechanics of absorption costing can lead to year-to-year income changes: Multiple Choice If the productivity of factory workers Improves. whenever Inventory levels remain fairly constant If...
-
Consider the portion of an excavator shown. At the instant under consideration, the hydraulic cylinder is extending at a rate of 6 in. /sec, which is decreasing at the rate of 2 in. /sec every...
-
Would water be a suitable proton source to protonate the following compound? ONa
-
When 3-bromo-3-ethylpentane is treated with sodium acetylide, the major products are 3-ethyl-2-pentene and acetylene. Explain why the carbon skeleton does not change in this case, and justify the...
-
Find the area of the region bounded by the given curves. tan?x, , 0
-
The financial statements for the Columbia Sportswear Company can be found in Appendix A, and Under Armour, Inc.'s financial statements can be found in Appendix B at the end of this book. Required a....
-
Use the data from SE3-8 to prepare the closing entries for The Decade Company. Close the temporary accounts to income summary. The balance of \(\$ 8,500\) in the retained earnings account is from the...
-
Adjusting Entries The following selected accounts appear in the Birch Company's unadjusted trial balance as of December 31, the end of the fiscal year (all accounts have normal balances): Required...
-
Closing Entries Use the information provided in E3-5A to prepare journal entries to close the accounts using the Income Summary account. After these entries are posted, what is the balance in the...
-
Ceva, Inc. manufactures and services jet engines for air carriers. The engines cost \($10\) to \($40\) million each, depending on the specifications and plane. A 10-year service contract for a single...
-
Solve each logarithmic equation in Exercises 4992. Be sure to reject any value of x that is not in the domain of the original logarithmic expressions. Give the exact answer. Then, where necessary,...
-
As indicated by mutual fund flows, investors tend to beat the market seek safety invest in last year's winner invest in last years loser
-
Explain why the reaction of the cis-isomer of this compound with potassium tert-but oxide in tert-butanol is about 500 times faster than that of thetrans-isomer. Br C(CH3)3
-
Explain which of these compounds has a faster rate of E2elimination: CH CH3 CI 'CI
-
Frequently, several different routes can potentially be used to synthesize a desired compound. For example, the following two routes can be envisioned for the preparation of cyclopentyl methyl ether....
-
Berbice Inc. has a new project, and you were recruitment to perform their sensitivity analysis based on the estimates of done by their engineering department (there are no taxes): Pessimistic Most...
-
#3) Seven years ago, Crane Corporation issued 20-year bonds that had a $1,000 face value, paid interest annually, and had a coupon rate of 8 percent. If the market rate of interest is 4.0 percent...
-
I have a portfolio of two stocks. The weights are 60% and 40% respectively, the volatilities are both 20%, while the correlation of returns is 100%. The volatility of my portfolio is A. 4% B. 14.4%...

Study smarter with the SolutionInn App


