Frequently, several different routes can potentially be used to synthesize a desired compound. For example, the following
Question:
Frequently, several different routes can potentially be used to synthesize a desired compound. For example, the following two routes can be envisioned for the preparation of cyclopentyl methyl ether. Explain which of these two routes you expect to give the higher yield of the desiredether.
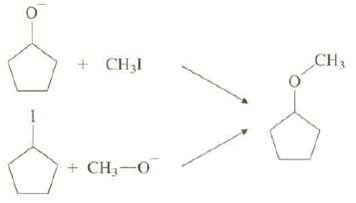
Transcribed Image Text:
CH3 + CH3I + CH3-O
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (11 reviews)
The upper route using the conjugate base of Cyclopentanol and methyl iodide wil...View the full answer

Answered By

Rukhsar Ansari
I am professional Chartered accountant and hold Master degree in commerce. Number crunching is my favorite thing. I have teaching experience of various subjects both online and offline. I am online tutor on various online platform.
5.00+
4+ Reviews
17+ Question Solved
Related Book For 

Question Posted:
Students also viewed these Organic Chemistry questions
-
Which of these compounds would you expect to be more soluble in water? Explain? CHCHCHCHCOH or CH3CHCHCHCHCOH
-
Which of these compounds would you expect to have the highest boiling point? Explain. [Section 24.4] CH3CH CH CH OH CHC=CH HCOCH
-
Show how Diels-Alder reactions might be used to synthesize the following compounds. (a) (b) (c) (d) (e) (f) (g) (h) (i) CH 3COOCH CH3 CN CN CI CI Cl Cl Cl CI CI C chlordane CI CI Cl Cl CI C aldrin CN...
-
Investment Portfolio and Risk diversification. Analyze the situations that arise in each of the problems and calculate the value or values ?? required for each of these. Problem 2: List and discuss...
-
Explain the difference between job costing and process costing. Give three examples (other than those in the chapter) of businesses that you think would use: (a) A job costing system (b) A process...
-
On October 1, 2017, Santana Rey launched a computer services company, Business Solutions that is organized as a proprietorship and provides consulting services, computer system installations, and...
-
Which of the following is not a process in project communications management? a. planning communications management b. controlling communications c. managing communications d. managing stakeholders...
-
A beginning accounting student tried to complete a work sheet for Dick Ady's Bookkeeping Service. The following adjusting entries were to have been analyzed and entered in the work sheet: (a) Ending...
-
Develop a network showing the origin nodes, destinations, arcs, cost per arc, supply, and demand. Match the correct answer to each question. Warehouse City E City F City G City H Warehouse Supply A...
-
Linda Butler is the new division controller of the snack-foods division of Daniel Foods. Daniel Foods has reported a minimum 15% growth in annual earnings for each of the past 5 years. The...
-
Explain which of these compounds has a faster rate of E2elimination: CH CH3 CI 'CI
-
What reagents and reaction conditions could be used to carry out the followingtransformations? Br - CH,CH,CH CH=CH, a) CH;CH,CH,CH;CH2 CH3 CH3 b) CH,CCI CH,COCH, CH3 CH3 C, Ph CH3 c) PHCH CI C=C CH3...
-
State whether each of the following is true or false. If false, explain why. a) An algorithm is a procedure for solving a problem in terms of the actions to execute and the order in which these...
-
If a change were made to Technical Spec 2 in the product's design, this would likely change the customer's opinion of which value feature the most? Quick Start Quick Start QFD Matrix 1 = Strong...
-
You are a quality management consultant for the Beserk Tennis Ball Company. Beserk is redesigning its current model of tennis ball, and you are asked to use QFD analysis to make suggestions about...
-
You are reviewing a tender evaluation that is to be awarded on lowest total price. The bid evaluations follow: To which company should the contract be awarded? Company Capital Cost Maintenance...
-
You have invited four companies to bid on a consulting project. All four companies answered your invitation to tender, but the bids vary in the number of hours each company estimates will be required...
-
Boston Cycles inventory data for the year ended December 31, 2011, follow: Assume that the ending inventory was accidentally overstated by $2,200. Requirement 1. What are the correct amounts for cost...
-
What are the chief characteristics of structured observation?
-
Identify the most stable compound:
-
What is probability of rolling an even number with 1 dice?
-
Predict the splitting pattern for each kind of hydrogen in isopropyl propanoate, CH3CH2CO2CH(CH3)2.
-
The acid-catalyzed dehydration of 1-methylcyclohexanol yields a mixture of two alkenes. How could you use 1H NMR to help you decide which waswhich? CH CH - 0* CH2
-
How could you use 1H NMR to distinguish between the following pairs ofisomers? (a) CH3CH=CHCH2CH3 and CH2 H H2H () CH20CH2CH and CH3OCH2CH2CH3 (c) CHC,H and CH3CH2CH3 (d) HCICH)H and CH3CH=CHCH3
-
The payroll register of Ruggerio Co. indicates $13,800 of social security withheld and $3,450 of Medicare tax withheld on total salaries of $230,000 for the period. Federal withholding for the period...
-
All of the following are included on Form 1040, page 1, EXCEPT: The determination of filing status. The Presidential Election Campaign check box. The income section. The paid preparer signature line.
-
Question One: (25 marks) (X) Inc. purchased 80% of the outstanding voting shares of (Y) for $360,000 on July 1, 2017. On that date, (Y) had common shares and retained earnings worth $180,000 and...

Study smarter with the SolutionInn App


