a. What is the general trend in first ionisation energies across Period 3? b. Explain why aluminium
Question:
a. What is the general trend in first ionisation energies across Period 3?
b. Explain why aluminium has a lower first ionisation energy than magnesium.
c. Explain why sulfur has a lower first ionisation energy than phosphorus.
d. Look at Period 4 in the Periodic Table. The first ionisation energies of the p-block elements are given in the table below. Predict the missing value for the first ionisation energy of selenium.
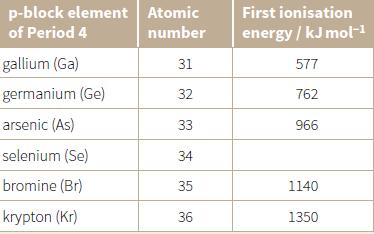
Transcribed Image Text:
p-block element Atomic First ionisation of Period 4 number energy / kJ mol-1 gallium (Ga) 31 577 germanium (Ge) 32 762 arsenic (As) 33 966 selenium (Se) 34 bromine (Br) 35 1140 krypton (Kr) 36 1350
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 90% (11 reviews)
a The general trend in first ionisation energies across Period 3 from sodium to argon is that the fi...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
a. Look at Figure 11.3. i. What is the general trend in the melting points going down Group 2? ii. Which element breaks the trend? b. Explain why the atoms in Group 2, as in any other group, get...
-
What is the general relationship between mortgage rates and long-term government security rates? Explain how mortgage lenders can be affected by interest rate movements. Also explain how they can...
-
What is the general relationship among operating leverage, financial leverage, and the total leverage of the firm? Do these types of leverage complement one another? Why or why not?
-
A line charge density pL of length L lies parallel to an infinite sheet of surface charge density ps. How much work is required to rotate the line charge so that it is vertical?
-
Professor Stone complains that student teacher ratings depend on the grade the student receives. In other words, according to Professor Stone, a teacher who gives good grades gets good ratings, and a...
-
Briefly describe the partial sale model of privatization. What is the predominant characteristic of the partial sale? How can a minority private investor try to protect itself from abuse by the...
-
What type of trend is indicated by the plot of the deseasonalized data? International Machinery, Inc., produces a tractor and wishes to use quarterly tractor sales data observed in the last four...
-
Scotch whisky increases in value as it ages, at least up to a point. For any period of time, t, the value of a barrel is given by V = 100t 6t2. This function implies that the proportional rate of...
-
Internal controls are not designed to safeguard assets from Select one: a. robbery. b. natural disasters. c. employee theft. d. unauthorized use. When an account becomes uncollectible and must be...
-
Consider the budgeted income statement for Carlson Company for June 20X4 in Exhibit 7-13. The cash balance, May 31, 20X4, is $15,000. Sales proceeds are collected as follows: 80% the month of sale,...
-
a i Describe how the atomic radius varies across Periods 2 and 3. ii. Explain this trend. b. i. Describe how the atomic radius varies down each group of the Periodic Table. ii. Explain this trend.
-
a. i. The Group 1 metal lithium reacts in a similar way to sodium. It reacts with oxygen, producing lithium oxide. Write the balanced symbol equation, including state symbols, for this reaction. ii....
-
What is the structure of a virus? Is a virus Irving? Explain how a virus is able to reproduce?
-
Tristan Walker of Walker & Company says, "We are only going to design, develop, and test products and services uniquely tailored to our community's needs. I get it. I'm a part of the community we are...
-
Ace Cosmetics Corporation purchased land adjacent to its plant to improve access for trucks making deliveries. Expenditures incurred in purchasing the land were as follows: purchase price, $55,000;...
-
7. At this point you now know information about both the horizontal and the vertical components of the projectile's velocity. In the space below, draw a diagram of the vector components of Vx and...
-
Complete autonomy in how you demonstrate the following criteria. In this module, we talked more about leadership. We discussed the differences between leadership theory which is a well-substantiated...
-
Accustart Ltd. acquired 38% of the common shares of Lecce Ltd. on January 1, 2024, by paying $5.76 million for 144,000 shares. Lecce declared a cash dividend of $0.60 per share in each quarter that...
-
Late in 2021, you and two other officers of Curbo Fabrications Corporation just returned from a meeting with officials of the City of Jackson. The meeting was unexpectedly favorable even though it...
-
Which property determines whether a control is available to the user during run time? a. Available b. Enabled c. Unavailable d. Disabled
-
Based on your answer to Problem 17.67, propose a mechanism for the following transformation: Answer Problem 17.67 Heat CO2 heat
-
Compare the structures of 1,4-pentadiene and divinyl amine: The first compound does not absorb UV light in the region between 200 and 400 nm. The second compound does absorb light above 200 nm. Using...
-
Provide a systematic name for each of the following compounds. a. b. c. d. e. H. CH3 Br
-
Los siguientes datos corresponden a las operaciones de Turk Company el ao pasado: Ventas $ 900 000 Utilidad operativa neta $ 36 000 Margen de contribucin $ 150 000 Activos operativos promedio $ 180...
-
Problem 16-16 Tax Shields (LO2) River Cruises is all-equity-financed with 53,000 shares. It now proposes to issue $280,000 of debt at an interest rate of 12% and to use the proceeds to repurchase...
-
In a process costing system, companies use predetermined overhead rates to apply overhead

Study smarter with the SolutionInn App


