Aluminium chloride, AlCl 3 , and ammonia, NH 3 , are both covalent molecules. a. i. Draw
Question:
Aluminium chloride, AlCl3, and ammonia, NH3, are both covalent molecules.
a. i. Draw a diagram of an ammonia molecule, showing its shape. Show any lone pairs of electrons.
ii. State the bond angle in the ammonia molecule.
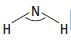
b. Explain why ammonia is a polar molecule.
c. An ammonia molecule and an aluminium chloride molecule can join together by forming a co-ordinate bond.
i. Explain how a co-ordinate bond is formed.
ii. Draw a dot-and-cross diagram to show the bonding in the compound formed between ammonia and aluminium chloride, H3NAlCl3. (Use a for a nitrogen electron, a for an aluminium electron and an for the hydrogen and chlorine electrons.)
d. Aluminium chloride molecules join together to form a compound with the formula Al2Cl6. Draw a displayed formula (showing all atoms and bonds) to show the bonding in one Al2Cl6 molecule.
Show the dative covalent bonds by arrows.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





