Electronegativity values can be used to predict the polarity of bonds. a. Explain the term electronegativity. b.
Question:
Electronegativity values can be used to predict the polarity of bonds.
a. Explain the term electronegativity.
b. The electronegativity values for some atoms are given below:
H = 2.1, C = 2.5, F = 4.0, Cl = 3.0, I = 2.5
Use these values to predict the polarity of each of the following bonds by copying the bonded atoms shown below and adding δ+ or δ– above each atom.
i. H—I
ii. F—I
iii. C—Cl
c. The shape of iodine trichloride, ICl3, is shown below.
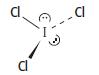
i. Describe the shape of this molecule.
ii. Explain why the ICl3 molecule has this shape.
iii. Suggest a value for the Cl — I — Cl bond angle.
d. The boiling points of the hydrogen halides are shown in the table.

i. Explain the trend in boiling points from HCl to HI.
ii. Explain why the boiling point of HF is so much higher than the boiling point of HCl.
e. Tetrachloromethane, CCl4, is a non-polar molecule.
i. Draw a diagram to show the shape of this molecule.
ii. Explain why this molecule is non-polar.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





