Benzene can be nitrated to give nitrobenzene. a. Name the mechanism for this reaction. b. The species
Question:
Benzene can be nitrated to give nitrobenzene.
a. Name the mechanism for this reaction.
b. The species attacking benzene in the reaction is NO2+. How is this generated in the reaction mixture?(Name the substances used and give a chemical reaction leading to the formation of NO2+.)
c. Suggest a suitable temperature for this reaction.
d. Use curly arrows to show the mechanism of how benzene reacts with NO2+ to produce nitrobenzene.
e. The structure of a common household substance is given below:
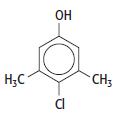
i. Give the molecular formula of the compound.
ii. Suggest a use for this compound in the home. Explain your suggestion.
iii. When bromine water is added to a solution of the compound, it is decolorised. Suggest two structures for the product of the reaction.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





