Bromate(V) ions react with bromide ions in acidic solution to form bromine. BrO 3 (aq) +
Question:
Bromate(V) ions react with bromide ions in acidic solution to form bromine.
BrO3–(aq) + 5Br –(aq) + 6H+(aq) → 3Br2(aq) + 3H2O(l)
a. Suggest two methods of following the progress of this reaction. For each method explain your answer.
b. The initial rates of reaction were compared using the initial concentrations of reactants shown in the table.
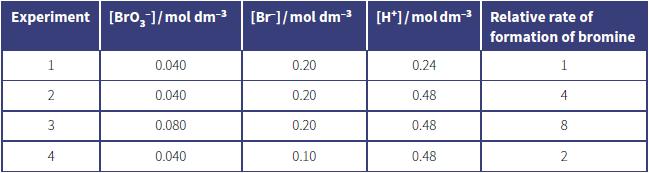
i. Deduce the order of reaction with respect to each reactant. In each case, show your reasoning.
ii. Deduce the rate equation for this reaction.
iii. State the units of the rate constant, k, for this reaction.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:




