Look at the elements in Period 2 of the Periodic TABLE. Using your knowledge of Period 3
Question:
Look at the elements in Period 2 of the Periodic TABLE. Using your knowledge of Period 3 elements, predict and explain the relative sizes of:
a. The atomic radii of lithium and fluorine.
b. A lithium atom and its ion, Li+.
c. An oxygen atom and its ion, O2−.
d. A nitride ion, N3−, and a fluoride ion, F−.
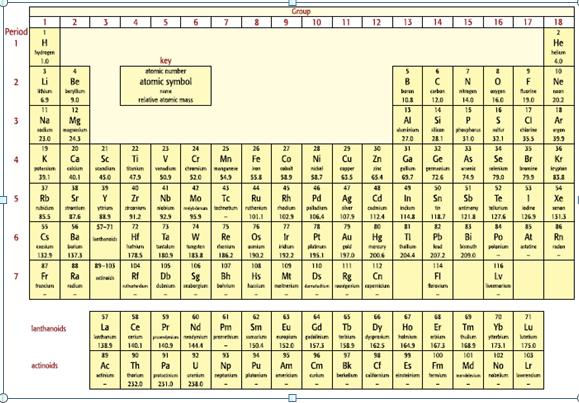
Transcribed Image Text:
2 3T4 10 11 12 13 14 15 16 17 18 Period key atomi umber 2 atomic elative atmic mas 3 4 6 lanthanoids Nd 1625 actinoids Th Np tharium pian cteium 232.0 2313 234.0 中 に . トz1 ala】 a2是 ||el. ト81a | |a a8|出】, a品| 1+p |よ =1コ12 aao
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 54% (11 reviews)
a The atomic radii of lithium Li and fluorine F are different due to the number of electrons and the ...View the full answer

Answered By

BillClinton Muguai
I have been a tutor for the past 5 years. I have experience working with students in a variety of subject areas, including computer science, math, science, English, and history. I have also worked with students of all ages, from elementary school to college. In addition to my tutoring experience, I have a degree in education from a top university. This has given me a strong foundation in child development and learning theories, which I use to inform my tutoring practices.
I am patient and adaptable, and I work to create a positive and supportive learning environment for my students. I believe that all students have the ability to succeed, and it is my job to help them find and develop their strengths. I am confident in my ability to tutor students and help them achieve their academic goals.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:
Students also viewed these Sciences questions
-
Group 2 of the periodic table contains the family of elements called the alkaline earths. How active chemically would you expect an alkaline earth element to be compared with the alkali metal next to...
-
The nitride ion and the amide ion, NH2, have greater attractions for the hydronium ion than the hydroxide ion does. Write the equations for the reactions that occur when calcium nitride and sodium...
-
Atomic lithium of concentration n = 3.6.1016 cm 3 is at a temperature T = 1500 K. In this case the power emitted at the resonant line's wavelength = 671 nm (2P 2S) per unit volume of gas is equal to...
-
Consider a long cylindrical solenoid with diameter R, number of current loops N and length L through which a current I runs. Now (a) Use Ampre's law to calculate the magnetic field inside the...
-
The following table shows ceremonial ranking and type of pottery sherd for a random sample of 434 sherds at a location in the Sand Canyon Archaeological Project, Colorado (The Architecture of Social...
-
What are the real economic impacts and long-term effects of trade sanctions? Assume that the United States imposes punishingly high tariffs of 100 percent on Japanese cars. Immediate costs might be...
-
The budget director of Feathered Friends Inc., with the assistance of the controller, treasurer, production manager, and sales manager, has gathered the following data for use in developing the...
-
During the current month, the following errors occurred in recording transactions in the purchases journal or in posting from it. a. An invoice for $1,875 of supplies from Kelly Co. was recorded as...
-
Please explain: 1. Benito bought a copy machine in March 2017 for $3,000. In May 2019 he exchanged the copy machine in a like-kind exchange for another copy machine with a FMV of $4,000. In June...
-
Dorina Company makes cases of canned dog food in batches of 1,000 cases and sells each case for $15. The plant capacity is 50,000 cases; the company currently makes 40,000 cases. DoggieMart has...
-
a. Explain what is meant by the term periodic property. b. The graph shows how a periodic property varies when plotted against atomic number for Period 3 (sodium to argon). i. Identify the property....
-
The variation of melting point with atomic number for Periods 2 and 3 is shown in the graph below. a. Explain what we mean when we say melting point is a periodic property. b. Explain the following....
-
Use Thevenins Theorem to find Vo in the network shown 3 12V 2mA
-
The ratio of CEO pay to that of an average employee increased over a period of 50 years from 24:1 to 275:1. Is this increasing gap ethically sound, in your opinion? Should CEO pay be limited in any...
-
Suppose you are considering buying a machine that costs $7,000. It will generate revenues of $1,500 for the next 3 years, and then $1,000 for the following 5 years. What is the payback period of this...
-
National Bakery Limited is the main supplier of a variety of baked products to customers in Kingston. The company currently makes 25,000,000 a variety of baked products annually which uses baking...
-
Q1. Discuss the financial goal of a business. Ensure to provide an example of the inherent ethical challenges associated with the financial goal and or the financial management process. Using the...
-
Q1. How can companies use social media to do sentiment analysis? Describe the process. Give an example of a company that uses sentiment analysis to enhance relationships with customers. Q2. Describe...
-
At December 31, 2021, Newman Engineerings liabilities include the following: 1. $10 million of 9% bonds were issued for $10 million on May 31, 1999. The bonds mature on May 31, 2029, but bondholders...
-
For all of the following words, if you move the first letter to the end of the word, and then spell the result backwards, you will get the original word: banana dresser grammar potato revive uneven...
-
Predict which side of the following equilibrium is favored, and explain your choice. Heat
-
When trans-3,4-dimethylcyclobutene is heated, conrotatory ring opening can produce two different products, yet only one is formed. Draw both products, identify which product is formed, and then...
-
Predict the product for each of the following reactions: (a) (b) (c) Heat Heat
-
Jennifer purchased a home for $1,000,000 in 2016. She paid $200,000 cash and borrowed the remaining $800,000. This is Jennifer's only residence. Assume that in year 2024, when the home had...
-
business plan describing company with strengths and weaknesses. Any gaps in plan. Recommendations for improvement of the plan.
-
You wish to buy a car today for $35,000. You plan to put 10% down and finance the rest at 5.20% p.a. for six years. You will make equal monthly payments of $_______.

Study smarter with the SolutionInn App


