Look at the Periodic Table in Figure 10.2. Figure 10.2 a. Which element is found in Period
Question:
Look at the Periodic Table in Figure 10.2.
Figure 10.2
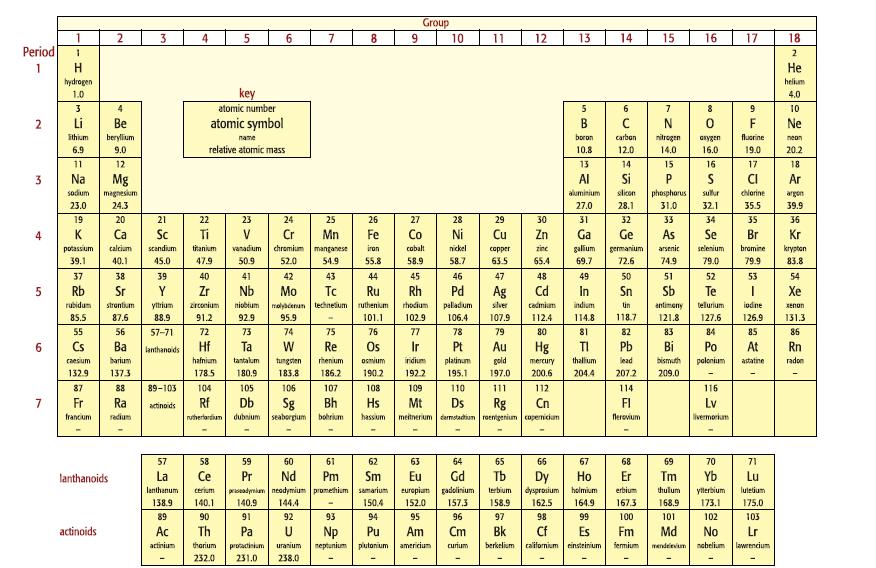
a. Which element is found in Period 4, Group 17?
b. The relative atomic masses of tellurium (Te) and iodine (I) are 128 and 127, respectively. Why did this present a problem to Mendeleev when he was constructing his Periodic Table?
c. Why are the elements in Groups 1 and 2 known as s-block elements, whereas those in Group 17 are p-block elements?
Group 9. 1 2 3 6. 7 8. 10 11 12 13 14 15 16 17 18 Period 2 Не hydrogen helium key 1.0 4.0 3 atomic number 9 10 2 Li Be atomic symbol B F. Ne ithium berylium boron carban nitrogen fluorine name arygen neon 6.9 9.0 relative atomic mass 10.8 12.0 14.0 16.0 19.0 20.2 11 12 13 14 15 17 18 3 Na Mg Al Si Ar sodum magnesium aluminium slicon phosphorus sullur chlerine argen 23.0 24.3 27.0 28.1 31.0 32.1 35.5 39.9 19 20 21 22 23 24 25 26 27 28 29 30 31 32 33 34 35 36 4 K Sc Mn Co Ni Cu Zn Ga Ge As Br Kr calcium scandium titanium vanadium chromium manganese iron cobalt nickel capper zine galium germanium arsenic selenium bramine kryptan 83.8 potassium 39.1 40.1 45.0 47.9 50.9 52.0 54.9 55.8 58.9 58.7 63.5 65.4 69.7 72.6 74.9 79.0 79.9 37 38 39 40 41 42 43 44 45 46 47 48 49 50 51 52 53 54 Rb Nb Mo To Ru Pd Ag Cd Sn Sb Te Хе rubidum zrconium molyacenan techneium ruthenium 95.9 strontium yttrium nisbium rhodium peladium slver cadmiam indium in antimeny tellurium iodine Xenon 85.5 87.6 88.9 91.2 92.9 101.1 102.9 106.4 114.8 118.7 121.8 127.6 126.9 131.3 55 56 57-71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 6. Cs Ba lanthanoids Hf Re Au Hg Pb Rn caesium barium hafnium tantalum tungsten rhenium Osmium iridium platinum gold mercury thallum lead bismuth polonium astatine radon 132.9 1373 178.5 180,9 183.8 186.2 190.2 192.2 195.1 197.0 200.6 204.4 207.2 209.0 87 88 89-103 104 105 106 107 103 109 110 111 112 114 116 Fr Ra Db Sg Bh Hs Rg meitnerium darmotadium roenigenium copemicam actinoids Mt Ds Cn FI Lv francium Tedium rutherlardium dubnium seaborgium bohrium hassium flerovium livermorium 57 58 59 60 61 62 63 54 65 66 67 68 69 70 71 La Ce Pr Nd Pm Sm Er Eu Gd europium gadolinium Tb Dy dysprosium lanthanoids Но Tm Yb Lu lanthanum priseadymiam neadymium promethium samarium 144.4 cerium terbium holmium erbium thulum yllerbium luletium 138.9 140.1 140.9 150.4 152.0 157.3 158.9 162.5 164.9 167.3 168.9 173.1 175.0 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103 Th Np neptunium plutonium Cm Bk Es californium einsteinium actinoids Ac Pa Pu Am Fm Md No Lr actinium tharium protacinium uranium americium curium berkelium fermium mendeievium nebelium lawrencium 232.0 231.0 238.0 O P. co
Step by Step Answer:
a The element found in Period 4 Group 17 is bromine Br bromine Br b On the modern periodic tabl...View the full answer



Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Related Video
The experiment aims to show the impact of various beverages on teeth by using eggs as a representation of enamel. Three eggs are boiled and then placed in glasses filled with fizzy drinks, vinegar, and mango juice for 24 hours. The shells of eggs are similar to enamel as they are composed of calcium carbonate, and enamel is primarily made of calcium phosphate. The eggs are then observed to demonstrate the effects of the different liquids on teeth and the importance of brushing regularly. The egg placed in fizzy drink has turned dark in color but can be cleaned by brushing with toothpaste and rinsing with water. The egg placed in vinegar has had its shell softened due to the chemical reaction of vinegar and calcium carbonate, which can\'t be reversed. This highlights the fact that acids are more damaging to teeth than other substances. The egg placed in mango juice represents the process of bacteria in the mouth converting sugars and starches into acids that form plaque, which can be prevented by brushing. The use of fluoride in toothpaste is also highlighted as it slows down the demineralization process and protects the enamel. The importance of brushing teeth twice a day is emphasized.
Students also viewed these Sciences questions
-
You own a call option on Intuit stock with strike price of $44. The option will expire in exactly three months' time. a. If the stock is trading at $52 in three months, what will be the payoff of the...
-
Why are the atomic masses of many elements (see the periodic table) not close to whole numbers?
-
Elements in group 7A in the periodic table are the halogens; elements in group 6A are called the chalcogens. (a) What is the most common oxidation state of the chalcogens compared to the halogens?...
-
Instructions In simplified form, you are going to be producing a script. The script will create 2 tables, load the 2 tables with data and then using PL/SQL it will process those 2 tables and with 2...
-
The following table shows the Myers-Briggs personality preference and area of study for a random sample of 519 college students (Applications of the Myers-Briggs Type Indicator in Higher Education,...
-
Hambro Bank, Ltd., an English bank, received a cable from a Danish company, A.O., requesting that an irrevocable letter of credit be opened in favor of J. H. Rayner and Company. A.O. instructed...
-
Table 29.4 lists the electron configurations of various atoms in their ground (lowest energy) states. The configuration notation can also describe atoms in their excited states. Which of the...
-
Identifying product and period costs Required Indicate whether each of the following costs is a product cost or a period (selling and administrative) cost. a. Advertising expense. b. Insurance on...
-
Question 2. Use the General Journal provided to record all the necessary entries for the disposal of the asset using double entry rules: Wildfire Traders sold office equipment on 31/10/18 for $6,600...
-
Purse Corporation owns 70 percent of Scarf Companys voting shares. On January 1, 20X3, Scarf sold bonds with a par value of $600,000 at 98. Purse purchased $400,000 par value of the bonds; the...
-
The activation energy for the uncatalysed decomposition of ammonia to its elements is +335 kJ mol 1 . a. Write the equation for this reaction, including state symbols. b. The enthalpy of reaction for...
-
a. Explain what is meant by the term periodic property. b. The graph shows how a periodic property varies when plotted against atomic number for Period 3 (sodium to argon). i. Identify the property....
-
Tresler Co. owns equipment that cost $92,500, with accumulated depreciation of $54,000. Tresler sells the equipment for cash. Record the sale of the equipment assuming Tresler sells the equipment...
-
Shire Company's predetermined overhead rate is based on direct labor cost. Management estimates the company will incur $649,000 of overhead costs and $590,000 of direct labor cost for the period....
-
You plan to live 25 years after you retire. You want to withdraw $100,000 each year for 25 years. Your first withdrawal will take place the day after you retire. What is the four annuity formulas...
-
Harwood Company's quality cost report is to be based on the following data: 2021 2022 Depreciation of test equipment $94,000 $95,000 Audits of the effectiveness of the quality system $54,000 $51,000...
-
Cash contribution of 4,000 to the Accounting Society (a charity) Purchase of art object at an Accounting Society Charitable event for $1,200 (FMV $800) Donation of 3-year-old clothing (basis 800; FMV...
-
The government is issuing $100 million in 10 year debt and receives the following bids. $25 million is reserved for non-competitive tenders. At what yield will the non-competitive tenders be issued...
-
Cupola Awning Corporation introduced a new line of commercial awnings in 2021 that carry a two-year warranty against manufacturers defects. Based on their experience with previous product...
-
Rewrite the code of Figure 7.3 in Ada, Java, or C#. Figure 7.3: template class queue { item items [max_items]; int next_free, next_full, num_items; public: queue () : next_free (0), next_full(0),...
-
Which of the following compounds below do you expect to have a longer λ max ?
-
Predict the expected λ max of the following compound:
-
When 5-deutero-5-methyl-1,3-cyclopentadiene is warmed to room temperature, it rapidly rearranges, giving an equilibrium mixture containing the original compound as well as two others. Propose a...
-
Marie Forleo, a marketing trainer and host of MarieTV, presents the eight tips for genuine networking. Do you agree or disagree with her suggestions? Discuss how this information is useful to you and...
-
Identify all relevant costs or revenue that are applicable to production- constrained decisions 1. Contributions margin of product 2. Interference with other production 3. Contribution margin per...
-
Gammaro Compary manufactures wallets from fabric. In 2 0 1 9 , Gammaro made 2 , 1 5 0 , 0 0 0 wallets using 1 , 2 5 0 , 0 0 0 yards of fabric. In 2 0 1 9 , Gammaro has capacity to make 2 , 8 0 0 , 0...

Study smarter with the SolutionInn App


