The diagram shows the enthalpy changes when sodium chloride is dissolved in water. a. Define the following
Question:
The diagram shows the enthalpy changes when sodium chloride is dissolved in water.
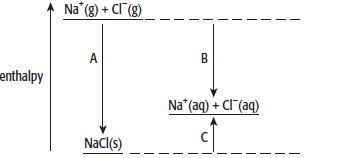
a. Define the following terms:
i. Enthalpy change of solution
ii. Enthalpy change of hydration.
b. Write symbol equations that describe the following:
i. Enthalpy change of solution of sodium chloride
ii. Enthalpy change of hydration of the chloride ion.
c. Name the enthalpy changes labelled a, B and C.
d. Draw the water molecules around magnesium ions and sulfate ions in a solution of magnesium sulfate.
e. Explain, in terms of differences of lattice energies and enthalpy changes of hydration, why magnesium sulfate is more soluble in water than calcium sulfate.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





