The information below gives the data for the reaction of hydrogen with iodine at 500C. H 2
Question:
The information below gives the data for the reaction of hydrogen with iodine at 500°C.
H2(g) + I2(g) ⇋ 2HI(g)
The table shows the initial partial pressures and the partial pressures at equilibrium of hydrogen, iodine and hydrogen iodide. The total pressure was constant throughout the experiment.
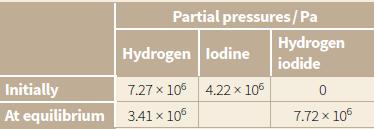
a. Deduce the partial pressure of the iodine at equilibrium.
b. Calculate the value of Kp for this reaction, including the units.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris
Question Posted:





