Water is extensively hydrogen bonded. This gives it anomalous (peculiar) properties. a. Explain why ice is less
Question:
Water is extensively hydrogen bonded. This gives it anomalous (peculiar) properties.
a. Explain why ice is less dense than liquid water.
b. State two other anomalous properties of water.
c. Propanone has the structure shown below.
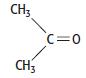
When propanone dissolves in water, it forms a hydrogen bond with water.
i. What features must water and propanone molecules posses in order to form a hydrogen bond?
ii. Draw a diagram to show a propanone molecule and a water molecule forming a hydrogen bond.
d. Propanone has a double bond. One of the bonds is a σ bond (sigma bond). The other is a π bond (pi bond).
i. Explain the difference between a σ bond and a π bond in terms of how they are formed.
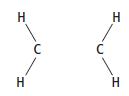
ii. Copy the diagram, then complete it to show the shapes of the electron clouds in the σ bond and the π bond between the carbon atoms in ethene. Label your diagram.
Step by Step Answer:

Cambridge International AS And A Level Chemistry Coursebook
ISBN: 9781316637739
2nd Edition
Authors: Lawrie Ryan, Roger Norris





