Question: a. For the adiabatic, steady-flow flash process from specified initial conditions of T 1 and P 1 to a specified final pressure P 2 shown
a. For the adiabatic, steady-flow flash process from specified initial conditions of T1 and P1 to a specified final pressure P2 shown here, develop the equilibrium and balance equations to compute the final temperature, the vapor-liquid split, and the compositions of the coexisting phases.
b. Develop an algorithm for the calculation of part (a) using an equation of state.
c. Use the Peng-Robinson equation of state for a multicomponent mixture to do the calculations for an adiabatic (Joule-Thomson) expansion of a liquid under pressure to produce a vapor-liquid mixture at ambient pressure. The results should include the outlet temperature, the mole fractions of each species in each phase, and the fractions of the outlet stream that are liquid and vapor.
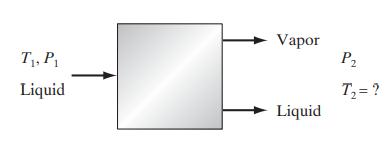
T, P Liquid Vapor Liquid P T = ?
Step by Step Solution
3.47 Rating (163 Votes )
There are 3 Steps involved in it
Part a Equilibrium and Balance Equations Consider an adiabatic steadyflow flash process from specified initial conditions of T1 and P1 to a specified final pressure P2 The system is assumed to be clos... View full answer

Get step-by-step solutions from verified subject matter experts


