Question: (a) Find the pH of a solution prepared by dissolving 1.00 g of glycine amide hydrochloride (Table 8-2) plus 1.00 g of glycine amide in
(a) Find the pH of a solution prepared by dissolving 1.00 g of glycine amide hydrochloride (Table 8-2) plus 1.00 g of glycine amide in 0.100 L.
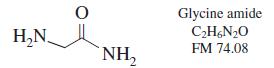
(b) How many grams of glycine amide should be added to 1.00 g of glycine amide hydrochloride to give 100 mL of solution with pH 8.00?
(c) What would be the pH if the solution in part (a) were mixed with 5.00 mL of 0.100 M HCl?
(d) What would be the pH if the solution in part (c) were mixed with 10.00 mL of 0.100 M NaOH?
(e) What would be the pH if the solution in part (a) were mixed with 90.46 mL of 0.100 M NaOH? (This is exactly the quantity of NaOH required to neutralize the glycine amide hydrochloride.)
Glycine amide C;H&N20 H,N. FM 74.08 NH,
Step by Step Solution
3.35 Rating (164 Votes )
There are 3 Steps involved in it
Answer a The pH of the solution prepared by dissolving 100 g of glycine amide hydrochloride Table 82 plus 100 g of glycine amide in 0100 L is 346 To calcu late the pH o f t his so lu t ion w e fi rst ... View full answer

Get step-by-step solutions from verified subject matter experts


