Question: a. In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. How many degrees of freedom are there for such
a. In vapor-liquid equilibrium in a binary mixture, both components are generally present in both phases. How many degrees of freedom are there for such a system?
b. The reaction between nitrogen and hydrogen to form ammonia occurs in the gas phase. How many degrees of freedom are there for this system?
c. Steam and coal react at high temperatures to form hydrogen, carbon monoxide, carbon dioxide, and methane. The following reactions have been suggested as being involved in the chemical transformation:
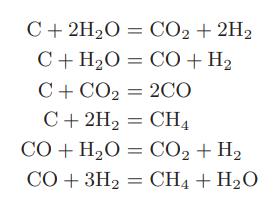
How many degrees of freedom are there for this system?
= CO+H, C + 2HO = CO + 2H C+H,O C+CO2 = 2CO C + 2H = CH CO+H,O = CO,+H, CO+ 3H CH4 + HO =
Step by Step Solution
3.46 Rating (153 Votes )
There are 3 Steps involved in it
a In vaporliquid equilibrium in a binary mixture both components are generally present in both phase... View full answer

Get step-by-step solutions from verified subject matter experts


