Question: Compute the difference between the pure-component and partial molar enthalpies for both components at 298.15 K and various compositions in each of the following mixtures
Compute the difference between the pure-component and partial molar enthalpies for both components at 298.15 K and various compositions in each of the following mixtures using the data in Fig. 8.1-2b.
a. Benzene–C6F5H
b. Benzene–C6F6
c. Benzene–C6F5Cl
d. Benzene–C6F5Br
e. Benzene–C6F5I
Fig. 8.1-2b
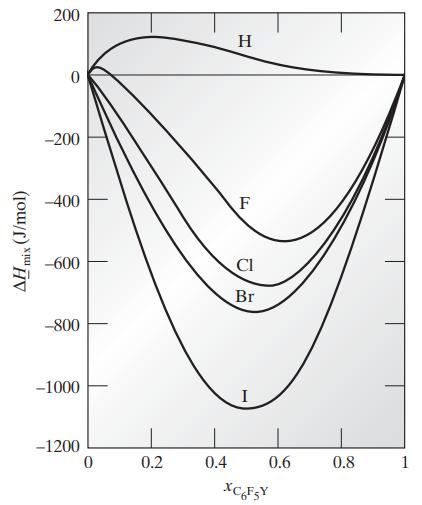
(J/mol) mix , 200 -200 -400 -600 -800 -1000 -1200 0 0.2 0.4 H F Cl Br 0.6 XC6FsY 0.8 1
Step by Step Solution
3.37 Rating (156 Votes )
There are 3 Steps involved in it
Tocompute the difference between the purecomponent and partial molar enthalpies for both components at 29815 K and various compositions in each of the ... View full answer

Get step-by-step solutions from verified subject matter experts


