a. Make the best estimate you can of the composition of the vapor in equilibrium with a
Question:
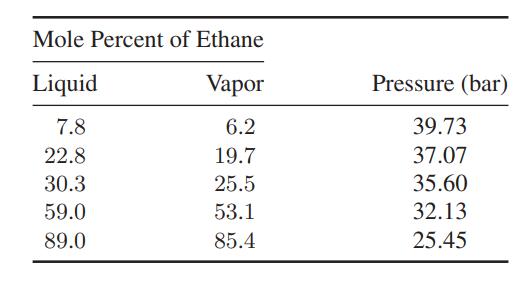
a. Make the best estimate you can of the composition of the vapor in equilibrium with a liquid containing 30.3 mol % ethane and 69.7 mol % ethylene at −0.01°C. Compare your results with the experimental data in the table.
b. Repeat the calculation in part (a) at other compositions for which the experimental data below are available.
Transcribed Image Text:
Mole Percent of Ethane Liquid Vapor 7.8 6.2 22.8 19.7 30.3 25.5 59.0 53.1 89.0 85.4 Pressure (bar) 39.73 37.07 35.60 32.13 25.45
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 75% (4 reviews)
a Calculate the composition of the vapor in equilibrium with a liquid containing 303 mol ethane and 697 mol ethylene at 001C To estimate the compositi...View the full answer

Answered By

Charan Mandhu
From my pre university course onwards i am teaching the students and online tutoring.I am about to complete my electronics in 1 months and i am good at computer science as well electrical iam capable to teach these subjects wherever student wants.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Redo Problem 10.3-7 using Aspen Plus. Problem 10.3-7 a. Make the best estimate you can of the composition of the vapor in equilibrium with a liquid containing 30.3 mol % ethane and 69.7 mol %...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
Track Rack is a software development company that specializes in developing information systems to account for inventory. The company maintains an attractive website, which is one of its primary...
-
A trainee in a medical lab will be released to work on her own when her results agree with those of an experienced worker at the 95% confidence level. Results for a blood urea nitrogen analysis are...
-
A summary of revenues and expenses for Stanton Company for 2008 follows: Sales . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . $6,000,000 Cost of goods manufactured and sold ....
-
Describe why audit assertions are important in the determination of audit risk.
-
Visit a local area firm to study how knowledge is being managed there at present, examine whether Web 2.0 technologies are being used, and talk to some of its people regarding how knowledge...
-
On July 1, Guy Fernandez established Fernandez Home Appraisal Services, a firm that provides expert residential appraisals and represents clients in home appraisal hearings. INSTRUCTIONS Analyze the...
-
Prepare a multi-step income statement, statement of retained earnings, & classified balance sheet and submit it for grading using Excel, Word, or a scanned handwritten copy. Be sure that the balance...
-
The General Social Survey reported in 2012 that in a sample of 68 men aged 18-25, the mean number of hours of television watched per day was 2.76 with a standard deviation of 2.21. In a sample of 72...
-
Vapor-liquid equilibria in petroleum technology are usually expressed in terms of K factors K i = y i /x i , where y i and x i are the mole fractions of species i in the vapor and liquid phases,...
-
a. Develop an algorithm for the equation-of-state prediction of the dew point pressure. b. Develop an algorithm for the equation-of-state prediction of the dew point temperature.
-
Use a calculator to find an approximation to four decimal places for each logarithm. ln 144,000
-
A particular brand of tires claims that its deluxe tire averages at least 50,000 miles before it needs to be replaced. From past studies of this tire, the standard deviation is known to be 8,000. A...
-
Quell Company operates three segments. Income statements for the segments imply that profitability could be improved if Segment A were eliminated. Required: a. Explain the effect on profitability if...
-
Lei, an accounting major, and Jim, a marketing major, are watching a Matlock rerun on latenight TV. Of course, there is a murder and the suspect wants to hire Matlock as the defense attorney. Matlock...
-
The following events apply to 2011, the first year of operations of Shay Services: 1. Acquired \(\$ 25,000\) cash from the issue of common stock. 2. Paid \(\$ 18,000\) cash in advance for a one-year...
-
Refer to the information for Gotta Cotta in PB 10-5. Required: Compute the following for Gotta Cotta: 1. Fixed overhead spending variance 2. Fixed overhead volume variance. 3. Over or underapplied...
-
Repeat Problem 15 using the universal JFET bias curve. In problem 15 For the network of Fig. 7.92, determine: 16 V 2.2 k 1a DSs6 mA DS 2.2 k 6-4 V
-
Distinguish among total-moisture content, free-moisture content, equilibrium-moisture content, unbound moisture, and bound moisture.
-
Find the pH of 0.050 M sodium butanoate (the sodium salt of butanoic acid, also called butyric acid).
-
The pH of 0.10 M ethylamine is 11.82. (a) Without referring to Appendix G, find Kb for ethylamine. (b) Using results from part (a), calculate the pH of 0.10 M ethylammonium chloride.
-
Which of the following bases would be most suitable for preparing a buffer of pH 9.00? (i) NH 3 (ammonia, K b = 1.76 10 -5 ); (ii) C 6 H 5 NH 2 (aniline, K b = 3.99 10 -10 ); (iii) H 2 NNH 2...
-
5. Discuss historical performance analysis. Address the methodology, areas to be evaluated and the corresponding ratios to the area. Describe how ratios are evaluated. That is, what are the ways we...
-
In a decision-making process, it is important for managers to distinguish between gross margin and contribution margin. List the ways in which these two margins differ.
-
Your task is record selected transactions for Sherby Corporation for 2021 and prepare year-end financial statements. Attached is a preliminary trial balance for Sherby. You can assume all...

Study smarter with the SolutionInn App


