Question: Redo Problem 10.3-7 using Aspen Plus. Problem 10.3-7 a. Make the best estimate you can of the composition of the vapor in equilibrium with a
Redo Problem 10.3-7 using Aspen Plus.
Problem 10.3-7
a. Make the best estimate you can of the composition of the vapor in equilibrium with a liquid containing 30.3 mol % ethane and 69.7 mol % ethylene at −0.01°C. Compare your results with the experimental data in the table.
b. Repeat the calculation in part (a) at other compositions for which the experimental data below are available.
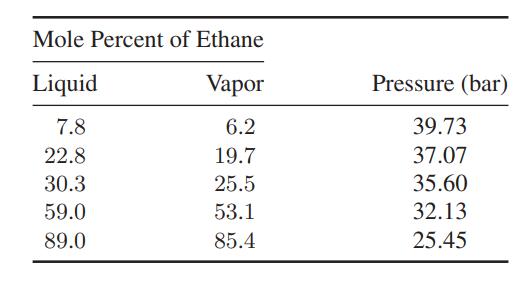
Mole Percent of Ethane Liquid Vapor 7.8 6.2 22.8 19.7 30.3 25.5 59.0 53.1 89.0 85.4 Pressure (bar) 39.73 37.07 35.60 32.13 25.45
Step by Step Solution
3.37 Rating (163 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


