A piston-and-cylinder device contains 10 kmol of npentane at 35.5C and 100 bar. Slowly the piston is
Question:
A piston-and-cylinder device contains 10 kmol of npentane at −35.5°C and 100 bar. Slowly the piston is moved until the vapor pressure of n-pentane is reached, and then further moved until 5 kmol of the n-pentane is evaporated. This complete process takes place at the constant temperature of −35.5°C. Assume n-pentane can be described by the Peng-Robinson equation of state.
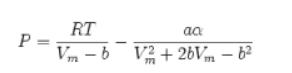 a. What is the volume change for the process?
a. What is the volume change for the process?
b. How much heat must be supplied for the process to be isothermal?
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





