A rough rule of thumb in polymer solution theory is that a 4 molar aqueous polymer solution
Question:
A rough rule of thumb in polymer solution theory is that a 4 molar aqueous polymer solution will have an osmotic pressure of approximately 100 bar. Is this rule of thumb in approximate agreement with Eq.11.5-5?
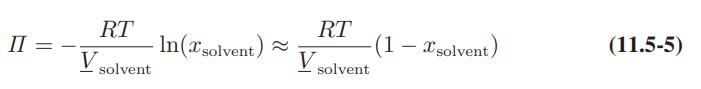
Transcribed Image Text:
II = RT V solvent -In(xsolvent) RT VSC solvent (1 - xsolvent) (11.5-5)
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 33% (3 reviews)
Yes the rule of thumb that a 4 molar aqueous polymer solution will have an osmotic pressure of appro...View the full answer

Answered By

PU Student
cost accounting
financial accounting
auditing
internal control
business analyst
tax
i have 3 years experience in field of management & auditing in different multinational firms. i also have 16 months experience as an accountant in different international firms. secondary school certification.
higher secondary school certification.
bachelors in mathematics.
cost & management accountant
4.80+
4+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
A rough rule of thumb is that light from the Sun reaches the Earth in about 8 minutes. Use this estimate along with the known speed of light to get an approximate value for the distance from the...
-
Insulin is a protein important in the metabolism of sugar. Its molar mass can be determined by means of an osmotic pressure experiment. A 50.0-mg sample of insulin was dissolved in enough water to...
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
Find the equation for the lower half of the circle x + (y-8) = 7. Put the equation in the form y = g(x), and then enter g(x) into the answer box below. Enter your answer as a symbolic function of x,...
-
On July 1, 2008, Baker Corporation sold equipment it had recently purchased to an unaffiliated company for $570,000. The equipment had a book value on Bakers books of $450,000 and a remaining life of...
-
Calculate the amount of bills receivable during the year. Opening balance of bills receivable Bill dishonoured Bills collected (honoured) Bills receivable endorsed to creditors Closing balance of...
-
How to diagnose an organizations training needs through the use of organizational, task, person, and demographic analyses
-
1. What are the ethical issues in this situation? Which issues must Berlex consider first when determining how to distribute Betaseron? 2. Given the shortage of the drug, how should Berlex decide who...
-
Sylvestor Systems borrows $165,000 cash on May 15 by signing a 90-day.7% $165,000 note 1. On what date does this note mature? 2 6. Prepare the entry to record issuance of the note. 2-b. First,...
-
This unadjusted trial balance is for Challenger Construction at the end of its fiscal year, September 30, 2014. The beginning balance of the owner's capital account was $46,000 and the owner invested...
-
Joe Udel lives on the second floor of a house that is adjacent to a well of pure water, but city water comes out of his indoor plumbing. He would rather have pure well water. So he has developed the...
-
Derive a form of the Gibbs phase rule that applies to osmotic equilibrium.
-
Lorrie places a 29-ft ladder against the side of a building with the bottom of the ladder 20 ft away from the building (see figure). How high up on the wall does the ladder reach? 29 ft ladder 20 ft
-
Under the race set up in Section8.1.2, think a scenario when you may have left truncation issue. 8.1.2 Truncation Another issue arising in the analysis of time to event data is truncation. Under...
-
Use mean score, IPW, and MI methods to estimate the sensitivity and specificity of the test in Example 11.1. Example 11.1 Suppose that we are interested in estimating the prevalence of a disease...
-
Assess the test-retest reliability for each of the domains of CSF-36 based on the study described in Example10.5. Example 10.5 Consider the random subsample of 197 patients in the CSF-36 study who...
-
To examine the quantity theory of money, Brumm (2005) ["Money Growth, Output Growth, and Inflation: A Reexamination of the Modern Quantity Theory's Linchpin Prediction," Southern Economic Journal,...
-
Consider the \(\operatorname{ARDL}(p, q)\) equation and the data in the file usmacro. For \(p=2\) and \(q=1\), results from the LM test for serially correlated errors were reported in Table 9.6 for...
-
Repeat Problem 34 if rd = 25 kΩ. In problem 34 Determine Zi Zo, and Av, for the network of Fig. 8.82 if rd = 60 kΩ. +22 V 1.8 k2 lass = 12 mA =-3.5 V Z. 10 100
-
The following T-accounts show postings of selected transactions. Indicate the journal used in recording each of these postings a through e. Cash Accounts Receivable Inventory (d) 500 (e) 300 (b)...
-
We have a 37.0 ( 0.5) wt% HCl solution with a density of 1.18 ( 0.01) g/mL. To deliver 0.050 0 mol of HCl requires 4.18 mL of solution. If the uncertainty that can be tolerated in 0.050 0 mol is 2%,...
-
Compute the molecular mass and its standard uncertainty for NH 3 . What is the percent relative uncertainty in molecular mass?
-
How many significant figures are there in the following numbers? (a) 1.903 0 (b) 0.039 10 (c) 1.40 10 4
-
XYZ Corp. applies manufacturing overhead costs to products at a budgeted indirect-cost rate of $65 per direct manufacturing labor-hour. A retail outlet has requested a bid on a special order of a...
-
What did you observe to be the major causes for the volatile week in stock trading this past week?
-
Analyze why there was underpricing or overpricing on listing price for Change Healthcare (CHNG)

Study smarter with the SolutionInn App


