Question: In a binary mixture, the activity coefficient of component 1 has been found to be where a, b, and are constants independent of temperature,
In a binary mixture, the activity coefficient of component 1 has been found to be
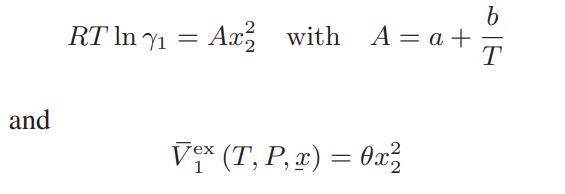
where a, b, and θ are constants independent of temperature, pressure, and composition. Find expressions for G, S, H, and V in terms of the gas constant R; the temperature T; the parameters a, b, and θ; the purecomponent properties; and the mixture composition.
and RT In 1 = 2 Ax with A = a + Vex (T, P, x) = 0x 1 79 T
Step by Step Solution
3.54 Rating (144 Votes )
There are 3 Steps involved in it
Finding an expression for G The Gibbs free energy of a binary mixture can be expressed as G Ge RTi x... View full answer

Get step-by-step solutions from verified subject matter experts


