In statistical mechanics one tries to find an equation for the partition function of a substance. The
Question:
In statistical mechanics one tries to find an equation for the partition function of a substance. The canonical partition function, Q(N,V,T), is used for a closed system at constant temperature, volume, and number of particles N. This partition function can be written as a product of terms as follows:
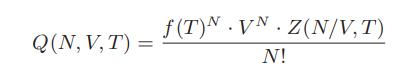
where f(T) is the part that depends only on the properties of a single molecule, and Z(N/V, T) is a normalized configuration integral that is unity for an ideal gas and depends on the interaction energies among the molecules for a real gas. Also, the Helmholtz energy is related to the canonical partition function as follows:
![]()
where k is Boltzmann’s constant (the gas constant divided by Avogadro’s number). Write expressions for all the thermodynamic properties of a fluid in terms of its canonical partition function and its derivatives.
Step by Step Answer:

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler





