Photosynthesis to produce sucrose C 12 H 22 O 11 can be considered to occur by the
Question:
Photosynthesis to produce sucrose C12H22O11 can be considered to occur by the following reaction
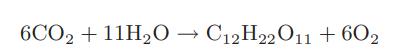
How much Gibbs free energy at 25°C must be obtained from sunlight for each mole of sucrose produced?
Transcribed Image Text:
6CO2 +11H₂O → C12H22011 +602
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 100% (4 reviews)
To determine the amount of Gibbs free energy obtained from sunlight for each mole of sucrose produced in photosynthesis we need to calculate the Gibbs ...View the full answer

Answered By

Ashington Waweru
I am a lecturer, research writer and also a qualified financial analyst and accountant. I am qualified and articulate in many disciplines including English, Accounting, Finance, Quantitative spreadsheet analysis, Economics, and Statistics. I am an expert with sixteen years of experience in online industry-related work. I have a master's in business administration and a bachelor’s degree in education, accounting, and economics options.
I am a writer and proofreading expert with sixteen years of experience in online writing, proofreading, and text editing. I have vast knowledge and experience in writing techniques and styles such as APA, ASA, MLA, Chicago, Turabian, IEEE, and many others.
I am also an online blogger and research writer with sixteen years of writing and proofreading articles and reports. I have written many scripts and articles for blogs, and I also specialize in search engine
I have sixteen years of experience in Excel data entry, Excel data analysis, R-studio quantitative analysis, SPSS quantitative analysis, research writing, and proofreading articles and reports. I will deliver the highest quality online and offline Excel, R, SPSS, and other spreadsheet solutions within your operational deadlines. I have also compiled many original Excel quantitative and text spreadsheets which solve client’s problems in my research writing career.
I have extensive enterprise resource planning accounting, financial modeling, financial reporting, and company analysis: customer relationship management, enterprise resource planning, financial accounting projects, and corporate finance.
I am articulate in psychology, engineering, nursing, counseling, project management, accounting, finance, quantitative spreadsheet analysis, statistical and economic analysis, among many other industry fields and academic disciplines. I work to solve problems and provide accurate and credible solutions and research reports in all industries in the global economy.
I have taught and conducted masters and Ph.D. thesis research for specialists in Quantitative finance, Financial Accounting, Actuarial science, Macroeconomics, Microeconomics, Risk Management, Managerial Economics, Engineering Economics, Financial economics, Taxation and many other disciplines including water engineering, psychology, e-commerce, mechanical engineering, leadership and many others.
I have developed many courses on online websites like Teachable and Thinkific. I also developed an accounting reporting automation software project for Utafiti sacco located at ILRI Uthiru Kenya when I was working there in year 2001.
I am a mature, self-motivated worker who delivers high-quality, on-time reports which solve client’s problems accurately.
I have written many academic and professional industry research papers and tutored many clients from college to university undergraduate, master's and Ph.D. students, and corporate professionals. I anticipate your hiring me.
I know I will deliver the highest quality work you will find anywhere to award me your project work. Please note that I am looking for a long-term work relationship with you. I look forward to you delivering the best service to you.
3.00+
2+ Reviews
10+ Question Solved
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:
Students also viewed these Engineering questions
-
Managing Scope Changes Case Study Scope changes on a project can occur regardless of how well the project is planned or executed. Scope changes can be the result of something that was omitted during...
-
The free-energy change (G') for the oxidation of the cytochrome aa3 complex by molecular oxygen is 102.3 kJ = 224.5 kcal for each mole of electron pairs transferred. What is the maximum number of...
-
(a)At 0 o C, liquid water and ice are in equilibrium at standard pressure, so they have the SAME molar Gibbs energy. When raise from 1.0 bar to 100 bar, how much Gibbs free energy change in the...
-
Graph the function y = (x + |x|). At what values of x does the derivative exist and what is the value of the derivative when it does exist?
-
Identify some possible causal factors for the following support departments: a. Cafeteria b. Custodial services c. Laundry d. Receiving, shipping, and stores e. Maintenance f. Personnel g. Accounting...
-
Montana Matts Golf Inc. was formed on July 1, 2014, when Matt Magilke purchased the Old Master Golf Company. Old Master provides video golf instruction at kiosks in shopping malls. Magilke plans to...
-
Why is the distinction between capital and revenue reserves so important for a company? L01
-
The stockholders' equities of Pal Corporation and Sip Corporation at January 1 were as follows (in thousands): On January 2, Pal issued 300,000 of its shares with a market value of $20 per share for...
-
Part 1 You are considering launching a Google AdWords campaign to promote your new Marketing Agency, "Luminous". Currently, your budget is $2,500 and you are curious to see what kind of results...
-
Another way to reduce the amount of nitric oxide is exhaust gasses is to inject ammonia resulting in the following reaction Determine the equilibrium conversion of nitric oxide at 1000C and 1 bar by...
-
An equimolar mixture of ethylene and hydrogen chloride react in the gas phase at 150C and 10 bar to form ethyl chloride. Determine the equilibrium composition at these conditions.
-
Net income was $500,000 in 2011, $450,000 in 2012, and $522,000 in 2013. What is the percentage of change from (a) 2011 to 2012 and (b) 2012 to 2013? Is the change an increase or a decrease?
-
Here are summary statistics for randomly selected weights of newborn girls: n = 36, x = 3180.6 g, s = 700.5 g. Use a confidence level of 99% to complete parts (a) through (d) below. a. Identify the...
-
2. (10 points) Two suppliers of products are available to supply the needs of four supermarkets. Each supplier can provide 90 units per day. Each supermarket would like to receive 60 units per day....
-
QUESTION 3 (11 marks) Midrand Ltd acquired a 90% interest in Bramely Ltd on 2 December 20.21 for R2 million. The consideration was settled as follows: Cash payment, Issue of 100 000 shares to the...
-
1. Prepare a Proforma Income Statement for ACCO 295 Corp. (30 points) Use the same Excel table provided to do the calculations with the class explanation. 1. Selling and administrative expenses were...
-
Sandy Foot Hospital expanded their cardiovascular unit to include more operating rooms. They negotiated a 20-year loan with monthly payments and a large sum of $250,000 due at the end of the loan....
-
Using PSpice, find the h parameters of the network in Fig. 19.123. Take = 1 rad/s O- +1F 2 I H
-
Which of the following statements is false? a. Capital leases are not commonly reported in a Capital Projects Fund. b. A governmental entity may report a Capital Project Fund in one year but not the...
-
Investigate whether the equilibrium can be maintained. The uniform block has a mass of 500 kg, and the coefficient of static friction is s = 0.3 200 mm A 600 mm 800 mm
-
The homogenous semicylinder has a mass of 20 kg and mass center at G. If force P is applied at the edge, and r = 300 mm, determine the angle at which the semicylinder is on the verge of slipping....
-
The uniform crate has a mass of 150 kg. If the coefficient of static friction between the crate and the floor is s = 0.2, determine whether the 85-kg man can move the crate. The coefficient of...
-
The principal amount of a bond is $65,000 its stated rate is 7%, and the term of the bond is 6 years. The bond pays interest semiannually. At the time of issue, the market rate is 8%. Determine the...
-
4) You make a deposit of $780 at 6% interest compounded annually. How much will be in the account in 3 years
-
This is the solved part A. Just need help in solving the next questions from 1-6. These four pages are interconnected one by one as i posted. First part was solved as I uploaded the picture...

Study smarter with the SolutionInn App


