Redo Problem 6.7 with the SoaveRedlich-Kwong equation of state. Problem 6.7 One hundred cubic meters of carbon
Question:
Redo Problem 6.7 with the Soave–Redlich-Kwong equation of state.
Problem 6.7
One hundred cubic meters of carbon dioxide initially at 150°C and 50 bar is to be isothermally compressed in a frictionless piston-and-cylinder device to a final pressure of 300 bar. Calculate
i. The volume of the compressed gas
ii. The work done to compress the gas
iii. The heat flow on compression assuming carbon dioxide
a. Is an ideal gas
b. Obeys the principle of corresponding states of Sec. 6.6
c. Obeys the Peng-Robinson equation of state
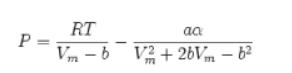
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemical Biochemical And Engineering Thermodynamics
ISBN: 9780470504796
5th Edition
Authors: Stanley I. Sandler
Question Posted:





